High Occurrence of Potentially Pathogenic Free Living Amoebae in Water Bodies of Kaleybar and Khodaafarin, East Azerbaijan Province
Hamed Behniafar1 , Maryam Niyyati1 * , Zohreh Lasjerdi1 and Samira Dodangeh1
DOI: http://dx.doi.org/10.12944/CWE.10.Special-Issue1.87
Free living amoebas are widespread protozoans that can be found in different environmental resources such as water bodies, soil and clays. Free living amoebae particularly Acanthamoeba could lead to diseases with poor prognosis including ameobic keratitis (AK) and granulomatous amebic encephalitis (GAE) especially in contact lens wears and immunocompromised individuals, respectively. In the present study 50 samples were collected from water resources (hot-springs, cold springs, rivers, wells, refined drinking water and subterranean canals) of Kaleybar and Khodaafarin, East Azarbayjan Province, Iran. The samples were transferred to laboratory and cultured on 1.5% non-nutrient agar medium containing Escherichia coli bacteria at room temperature and finally examined by microscopic methods. The results showed that out of 50 water samples, 40% were positive samples for free-living amoebae (FLA) including 60% Acanthamoebae genus, %35 Haetmannellidae, %20 Valkamphiids, %20 Vannella, %10 Thechamoeba (45% of plates had mix contamination). The present research was the first study to address the presence of potentially pathogenic free living amoebae in Kaleybar and Khodaafarin, East-Azerbaijan Province.
Copy the following to cite this article:
Behniafar H, Niyyati M, Lasjerdi Z, Dodangeh S. High Occurrence of Potentially Pathogenic Free Living Amoebae in Water Bodies of Kaleybar and Khodaafarin, East Azerbaijan Province. Special Issue of Curr World Environ 2015;10(Special Issue May 2015). DOI:http://dx.doi.org/10.12944/CWE.10.Special-Issue1.87
Copy the following to cite this URL:
Behniafar H, Niyyati M, Lasjerdi Z, Dodangeh S. High Occurrence of Potentially Pathogenic Free Living Amoebae in Water Bodies of Kaleybar and Khodaafarin, East Azerbaijan Province. Special Issue of Curr World Environ 2015;10(Special Issue May 2015). Available from: http://cwejournal.org?p=683/
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2014-11-25 |
|---|---|
| Accepted: | 2014-12-30 |
Introduction
Free living amoebae including Acanthamoeba, Hartmannella, Vahlkampfia, Vannella and Naegleria are small amphizoic protozoan parasites which feed on natural environment contents such as bacteria and fungi. These organisms are resistant to extreme environmental conditions including unfavorable temperature, pH ranges and exposure to chemicals in cyst stage and they transfer to trophozoite when condition became favorable (Stockman et al., 2011) (Pelletier et al., 2011)
Free living amoebae could be isolated from water resources, soil, sewage and even contact lens washing solution and dialysis machine(Marciano-Cabral et al.,2003). The most common cause of amoebic keratitis (AK) associated with Acanthamoeba is improper usage of soft contact lenses such as taking shower or swimming in suspected water sources while wearing lenses (Martinez AJ et al.,1997)( Radford CF et al.,1992). The mentioned infection causes severe eye pain, uveitis wounds, photophobia and blindness (Dart, 1993).
Unlike amoebic keratitis, granulomatous amebic encephalitis (GAE) has been reported only from immunocompromised individuals. Indeed, normal immune system could prevent the parasite pathogenesis. Main routes of parasite entrance in GAE are cysts inhalation and contact of skin wounds with contaminated environmental sources (Marciano-Cabral et al., 2003).
Although some drugs like biguanide, diamidine and triazoles are choices for both diseases, treatment is difficult and it fails in majority of cases. It should be mentioned that other free living amoebae such as Vahlkampfia and Hartmannella could be the cause of infection in both immunocompetent and immunosuppressed patients. Interestingly there are several case reports in Iran during recent years regarding Ak due to Vahlkampfia and Hartmannell(Niyyati M et al.,2010). It should be mentioned that due to increasing number of high risk individuals and extended spread of pathogenic FLA, outbreaks can be predicted in future. Overall, occurrence of free living amoebae in recreational water bodies may constitute a source of amoebae for high risk people including contact lens wearers and immunosuppressed patients.
Kaleybar and Khomarlou cities are the centers of Kaleybar and Khodaafarin counties, respectively which both located in East Azerbaijan Province, northwest of Iran. Kaleybar and Khodaafarin are located 1240 meters above sea level. Both counties together have a population of 83814 in 2012 census (48837 and 34977 respectively). The counties total area is 3702 km2. Kaleybar and Khodaafarin have a mountain climate with regular seasons.
Overall, there were no data regarding the occurrence of free living amoebae in water bodies in Kalaybar and Khodaafarin Counties thus the present research was conducted to clarify the presence of free living amoebae using morphological key. To the best of our knowledge this is the first report of occurrence of FLA in this region.
Material and Methods
Sampling
50 water samples (500–1500ml) were collected from different water resources of Kaleybar and Khodaafarin Counties in East Azarbayjan Province (Figure 1), in March and November 2014 including hot-springs (3 samples), cold-springs (29 samples), rivers (5 samples), wells (2 samples), refined drinking water (10 samples) and subterranean canal (1 sample). The samples were transferred to Protozoology laboratory, Shahid Beheshti University of Medical Science, Tehran, Iran and stored at room temperature.
 |
Figure 1: Star show Kaleybar and Khodaafarin position in East Azerbaijan province Click here to View figure |
Filtration and Cultivation
Approximately 250 ml of each sample filtered through cellulose nitrate membrane (pore size of membranes were equal 1.6µm).10 After filtering middle part of each membrane were cut out and cultured. Culturing conducted on 1.5% non-nutrient agar containing Escherichia coli bacteria. Plates were then incubated at room temperature up to two month.
Microscopic Examination and Cloning
Investigation of plates started after one week and continued daily up to two month. Positive samples were identified using light microscopy using x100 magnification. Cloning were then performed for elimination of bacteria and fungi contamination and also to isolate each clone of free living amoebae in mixed amoebae plates. Identification was based on page key and according to morphological criteria of each cloned amoebae.
Results
A total of 20 (%40) out of 50 samples were positive in microscopic examination. Isolated genera were belonged to Acathameobae genus (Figure 2 and 3), Thechameoba (Figure 4), Hartmannella (Figure 5), Valkamphiids and Vannella (Figure 6). Acanthamoeba were detected by its double walled shape and flat trophozoite with obvious acanthopodia. Hartmannellidae were identified by its wormy shape trophozoites and small round cysts. Vannella were characterized by its fan shaped trophozoites and Vahlkampfids had round cysts measuring approximately 10 micron, their trophozoite were long and wormy shape.
Number and percentage of positive water resources and isolated amoebae are shown in table1 and table 2, respectively.
Table 1: positive samples of water resource (number and percentage)
| Resource | Number of samples | Number of positive samples | Positive percentage |
| Cold spring | 29 | 11 | %38 |
| Refined drinking water | 10 | 3 | %30 |
| River | 5 | 4 | %80 |
| Hot-spring | 3 | 1 | %33 |
| Well | 2 | 1 | %50 |
| Subterranean canal | 1 | 0 | %00 |
| Total | 50 | 20 | %40 |
Table 2: Isolated free living amoeba from water samples and their percentage
| Amoebae genus | Number of positive samples | Percentage |
| Acanthamoebae | 12 | %60 |
| Thecameoba | 2 | %10 |
| Harmannella | 7 | %35 |
| Valkamphia | 4 | %20 |
| Vannella | 4 | %20 |
| Mix | 9 | %45 |
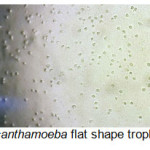 |
Figure 2: Acanthamoeba flat shape trophozoites (x100) Click here to View figure |
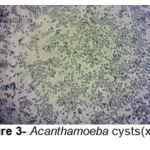 |
Figure 3: Acanthamoeba cysts(x100) Click here to View figure |
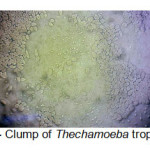 |
Figure 4: Clump of Thechamoeba trophozoites Click here to View figure |
 |
Figure 5: Hartmanellidae cysts (x100) Click here to View figure |
 |
Figure 6: Vannella with fan shape trophozoites (x100) Click here to View figure |
Discussion
Present study conducted to investigate the occurrence of free living amoeba in Kaleybar and Khodaafarin Counties water resources by culture method and microscopic investigation. The present study showed 20 out of 50 (%40) water samples were contaminated to potentially pathogenic free living amoebae. About half (9 out of 20) of samples had mix contamination and various amoebae were detected. As expected the frequency of Acanthamoeba species (12 samples out of 20 samples) were higher than others and the least occurance belonged to Thechameoba (2 samples out of 20 positive samples). In addition present study suggests that different kind of water resources (cold spring, hot spring, refined drinking water, river and well) were contaminated to at least one amoeba, but the highest contamination was seen in rivers water with 4 positive samples out of a total of 5 samples. Although the subterranean canal sample result was negative but because of the low sample number (1 sample) this result cannot be reliable.
Based on Bagheri et al. study, 45 samples out of 94 tap water samples (%48) were contaminated with Acanthamoeba.11 In this study samples were collected from tap water of hospitals in different cities of Iran. According to our study Acanthamoeba contamination was also higher in their research. High occurrence of Acanthamoeba is mainly due to its resistance to extreme environmental conditions. This level of resistance is contributed to morphological changes and development of cyst stage. Cyst form is resistant to a wide range of temperature, pH and osmalarity.
Ghadar-ghadr et al. reported %48 contamination of different kinds of water resources with free-living amoeba by using culturing, giemsa staining and microscopic investigation methods(Solhjoo K et al., 2012) Identified amoebae in their research were Naegleria and Acanthamoeba.
Nazar et al. determined %32 contamination with Acanthamoeba in water resources(Nazar M et al.,2011) Samples were collected from parks and public squares in this research. The mentioned study reported higher contamination to Acanthamoeba in comparison to present study, however they did not identify other free living amoebae in their samples. Another research in Spain revealed that 48 out of 88 (%59.5) tap water contained Acanthamoeba.
Other identified amoebae in the present research were Hartmannellidae and Vahlkampfiids both are causative agents of amoebic keratitis in Iran and worldwide.8,9 Keratitis due to Hartmannella and Vahlkampfia also showed poor prognosis and they could lead to loss of vision. Thus recreational water contamination to such amoebae could be a health hazard for high risk people and education to contact lens wears regarding avoidance of contact with suspected water is necessary.
As we see in present study free living amoebae can be found in different natural water resources associated with human activity in Kalaybar and Khodaafarin Counties with high frequency. This is the first study which showed water bodies contamination to free living amoebae in this region and this issue could reflect a potential hazard particularly for contact lens wearers and immunosuppressed patients. Posting of alarming sign in recreational water places could be an approached to prevent free living amoebae related infections. More researches should be implicated regarding molecular analysis of free living amoebae in this Counties.
Acknowledgment
Dr. Maryam Niyyati has been funded by young associated professor grant of Elite foundation.
Thanks are due to Mr. Kamandar Sheikhzadeh, Mr. Jabbar Dirineh, Mr. Seyed Ahmad Karamati and Mr. Amir Reza Javadi for their kind help in processing of the samples.
References
- Stockman LJ, Wright CJ, Visvesvara GS, Fields BS, Beach MJ. Prevalence of Acanthamoeba spp. and other free-living amoebae in household water, Ohio, USA—1990–1992. Parasitology research. 2011;108(3):621-7. (Stockman et al., 2011)
- Pelletier JS, Miller D, Liang B, Capriotti JA. In vitro efficacy of a povidone–iodine 0.4% and dexamethasone 0.1% suspension against ocular pathogens. Journal of Cataract & Refractive Surgery. 2011;37(4):763-6. (Pelletier et al., 2011)
- Khan NA, Tareen NK. Genotypic, phenotypic, biochemical, physiological and pathogenicity-based categorisation of Acanthamoeba strains. Folia parasitologica. 2003;50(2):97-104.
- Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clinical Microbiology Reviews. 2003;16(2):273-307.
- Martinez AJ, Visvesvara GS. Freeâ€living, amphizoic and opportunistic amebas. Brain Pathology. 1997;7(1):583-98.
- Radford CF, Lehmann OJ, Dart JK. Acanthamoeba keratitis: multicentre survey in England 1992–6. British journal of ophthalmology. 1998;82(12):1387-92.
- Dart J. Disease and risks associated with contact lenses. The British journal of ophthalmology. 1993;77(1):49.(Dart, 1993)
- Niyyati M, Lorenzo-Morales J, Rezaie S, Rahimi F, Martín-Navarro CM, Mohebali M, et al. First report of a mixed infection due to Acanthamoeba genotype T3 and Vahlkampfia in a cosmetic soft contact lens wearer in Iran. Experimental parasitology. 2010;126(1):89-90.
- Abedkhojasteh H, Niyyati M, Rahimi F, Heidari M, Farnia S, Rezaeian M. First Report of Hartmannella keratitis in a Cosmetic Soft Contact Lens Wearer in Iran. Iranian journal of parasitology. 2013;8(3):481.
- Rezaeian M, Niyyati M, Farnia S, Haghi AM. Isolation of Acanthamoeba spp. from different environmental sources. Iranian Journal of Parasitology. 2008;3(1):44-7.
- Bagheri H, Shafiei R, Shafiei F, Sajjadi S. Isolation of Acanthamoeba Spp. from drinking waters in several hospitals of Iran. Iranian journal of parasitology. 2010;5(2):19.
- Sh G-g, Solhjoo K, MJ N-n, Rohi R, Zia-Jahromi S. Isolation and identification of free living amoeba (Naegleria and Acanthamoeba) in Shiraz water resources by morphological criteria. Journal of Jahrom University of Medical Sciences. 2012;10(3):27.
- Nazar M, Haghighi A, Niyyati M, Eftekhar M, Tahvildar-Biderouni F, Taghipour N, et al. Genotyping of Acanthamoeba isolated from water in recreational areas of Tehran, Iran. Journal of water and health. 2011;9(3):603-8.
- Lorenzo-Morales J, Lindo J, Martinez E, Calder D, Figueruelo E, Valladares B, et al. Pathogenic Acanthamoeba strains from water sources in Jamaica, West Indies. Annals of tropical medicine and parasitology. 2005;99(8):751-8.







