Isolation and Characterization of Two Cyanobacterial Strains Calothrix Sp. and Microchaete Sp. from Rice Fields of Karimganj District, Assam, North East India
Moirangthem Thajamanbi 1 , Jayashree Rout 1 * and Nooruddin Thajuddin 2
Corresponding author Email: routjaya@rediffmail.com
DOI: http://dx.doi.org/10.12944/CWE.11.2.07
Studies on various nitrogen fixing microalgal strains found in the rice paddy field soils are carried out in different parts of the world. In the present study two cyanobacterial strains belonging to the order nostocales, Calothrix sp. and Microchaete sp. were isolated from the rice fields of Karimganj district, South Assam, India and characterized based on their morphological, biochemical and molecular analysis. For the phenotypic characterization - growth, pigments (chlorophyll a, total carotenoid content, phycobiliproteins) and biochemical properties (total carbohydrate and soluble proteins) were studied. The study showed that both strains contain lower phycoerythrin content as compared to the other pigments. The Microchaete strain contain a higher total carotenoid content while chlorophyll a accumulation was higher in the Calothrix strain. Phylogenetic compairision was made using 16S rRNA gene sequences including other sequences of Calothrix, Microchaete and Tolypothrix species from GenBank. The results showed that polyphasic approach provides necessary information for the identification of cyanobacterial species using morphological analysis in combination with molecular techniques.
Copy the following to cite this article:
Thajamanbi M, Rout J, Thajuddin N. Isolation and Characterization of Two Cyanobacterial Strains Calothrix Sp. and Microchaete Sp. from Rice Fields of Karimganj District, Assam, North East India. Curr World Environ 2016;11(2) DOI:http://dx.doi.org/10.12944/CWE.11.2.07
Copy the following to cite this URL:
Thajamanbi M, Rout J, Thajuddin N. Isolation and Characterization of Two Cyanobacterial Strains Calothrix Sp. and Microchaete Sp. from Rice Fields of Karimganj District, Assam, North East India. Curr World Environ 2016;11(2). Available from: http://www.cwejournal.org?p=952/
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2016-07-30 |
|---|---|
| Accepted: | 2016-08-21 |
Introduction
Cyanobacteria are oxygen evolving photoautotropic prokaryotes known to cohabitate with rice and exploited in agriculture for their specific inoculation as nitrogen supplementing biofertilizers in paddy fields.1 They have received much attention in soil due to their nitrogen fixing ability and significant contribution in primary production. The rice field ecosystem provides an environment favourable for the growth of cyanobacteria with respect to their requirements for light, water, high temperature and nutrient availability.2 The classification system of cyanobacteria usually depends on morphological attributes, which however, are not always consistent as they may show disparity with change in culturing conditions.3 Therefore a polyphasic approach involving traditional morphology, biochemical and molecular data has become necessary in recent years.
According to the traditional classification system, the genus Calothrix as described by C. Agardh (1824) belong to the order Nostocales and family Rivulariaceae.4-5 It is a polymorphic genus with the general features including hormogonia giving rise to young filaments with terminal heterocyst at only one end of the trichome, mature trichome tapers from base, which bears a terminal heterocyst to apex, vegetative cells disc-shaped, isodiametric or cylindrical.5 Recent studies have suggested that a polyphasic approach (using both morphological and molecular data) is required to determine Calothrix taxa.6 The Rivulariaceae are considered amongst the most morphologically complex cyanobacteria with tapered trichomes, apart from short phases of hormogonium formation that has a terminal heterocyst, although in some species intercalary heterocyst is also present and cell division is largely localized to a region near the heterocyst7. Species of the genus Calothrix are blue green, filamentous with a basal heterocyst and occur in both salt and freshwater environments as well as sub- aerially and aerially.8-9
The other genera Microchaete, [Thuret 1875] Bornet et Flahault 1886, is a filamentous, heterocystous cyanobacterium that belongs to the family Microchaetaceae, Division Cyanophyta generally found in ponds, rice paddy fields, fresh water lakes, and marine environments.10-12 Usually filaments are attached by one end to a substratum, single or with colonies of many filaments which are arranged irregularly or forming a turf. Trichome surrounded by a very distinct sheath, mostly narrow and tapered towards the end, heterocyst basal and also often intercalary. The two genera differ morphologically where cell division in Calothrix occurs mainly in the part of the trichome near the heterocyst while cell division in Microchaete apparently takes place towards the apex and cell elongation takes place away from this region.13 Therefore, the objective of the present study was to isolate the two cyanobacterial strains from the rice fields of Karimganj district, South Assam, India and characterize them for morphological, biochemical and molecular analysis.
Materials and Methods
Isolation of strains from soil samples
The soil samples were collected from selected rice fields of Karimganj district, South Assam. The strain Calothrix sp. (AUS-JR/MT/NT-036) was isolated from the rice field of Deodhar (24°69'862'' N, 92°45'115''E) and Microchaete sp.(AUS-JR/MT/NT-037) from the rice field of Sone beel (24°42'059''N, 92°27'164''E) respectively. Ten gram of the soil sample was transferred to a 250ml flask containing 90ml sterile distilled water and shaken (120 rpm) for 30 mins.14 Serial dilution (10-2, 10-1) was made and 1ml aliquots were spread on agar plates containing BG 11o N-free medium.15 The resulting axenic cyanobacterial isolates were maintained in their isolation media at 24°C±1 in laboratory conditions with a light intensity of 2000-3000lux. Repeated subculturing was performed a number of times until pure axenic culture were obtained.
Phenotypic characterization of the cyanobacterial isolates
The morphological characterization of the cyanobacterial isolates was determined. Microscopic observation and microphotography of the pure cultures were made with the aid of Leica DM1000 microscope and morphological characteristics such as structure and colour of thallus, filaments length and width; attenuation, shape and size of vegetative cells, constriction, position, shape and number of heterocysts, colour, thickness and distance of sheath, and presence or absence of spores. Identification of the strains was done using taxonomic keys.16
Growth characteristics and biochemical estimation
Growth pattern, biochemical constituents (soluble protein and total carbohydrate) and pigment profile (chlorophyll a, total carotenoid and phycobiliprotein) of the two cyanobacterial isolates were examined. The cyanobacterial growth pattern was determined by analyzing the species biomass in terms of chlorophyll a concentration.17 Soluble protein was measured with the modified Lowry method18 and total carbohydrate was estimated by the Anthrone method.19 Phycobiliproteins and total carotenoid content were also estimated.20-21
16S rDNA gene amplification and sequencing
Genomic DNA of the two isolated cyanobacterial strains was extracted from exponentially growing cultures following standard method.22 Amplification of 16S rDNA genes were done using CYA106 f (5’-GGACGGGTGAGTAACGCGTGA-3’) and CYA781r (a) (5’-GACTACTGGGGTATCTAATCCCATT-3’) as forward and reverse primers respectively in the Applied Biosystem thermal cycler with the following temperature as initial denaturation at 94°C for 5min, 30cycles of 94°C for 1min, 58°C for 45s, 72°C for 1min and final extension at 72°C for 7min23. The 50µl of reaction mixture was prepared using 2µl (50 ng) of extracted DNA and the PCR reaction mixture consisting of 2x Genet Bio Premix pH 9.0 (Prime Taq TM DNA Polymerase 1 unit 10 µl, 20 mM Tris-HCl, 80 mM KCl, 4 mM MgCl2, enzyme stabilizer sediment, loading dye and 0.5mM of each dATP, dCTP, dGTP, dTTP) 25 µl, 1µl of each forward and reverse primers, and 21µl MiliQ water.
Phylogenetic analysis and construction of tree
The obtained sequences were checked for homology with other sequences deposited in the available databases using Basic Local Alignment Search Tool (BLAST) search (http://www.ncbi.nim.nih.gov/BLAST). The gene sequences were then submitted to National Centre for Biotechnology Information (NCBI) GenBank, US., under their respective accession numbers. Phylogenetic tree was constructed using the MEGA6 analysis platform24 including the available cyanobacterial gene sequences along with the sequences determined in this study using the neighbour-joining method.25 Sequences were aligned using the CLUSTALW aligning utility to produced working alignment of 16S rDNA sequences for the target strains. The evolutionary distances were computed and expressed as number of base substitutions per site. Statistical significance level of interior nodes was determined by bootstrap analysis (1000 data re-samplings).26
Nucleotide accession numbers
The sequences of the 16S rRNA genes of the strains have been deposited in the NCBI Genbank and respective accession numbers were obtained.
Results and Discussion
Morphological characterization of strains
The morphology of the isolated cyanobacterial strains were studied under light microscope. The morphological features of the two strains are as follows.
Calothrix sp. (AUS-JR/MT/NT-036)
The structure of the thallus on petri plate was filamentous; blue green or brownish in colour; slightly bent, single, 7-10µm broad at the base with thin colourless sheath; trichome brownish green, constricted at the cross walls; cells quadratic, shorter or longer than broad, 4-7µm long and 3-7µm broad; heterocyst single, basal and spherical, 4-5µm broad. (Fig. 1 a, c and e).
Microchaete sp. (AUS-JR/MT/NT-037)
The culture on the petri plate were filamentous, blackish brown in colour, forming a wooly type thin film; trichomes solitary, slightly attenuated towards the end; cells quadrate 8-9µm broad, 9-11µm long; cell contents granular at the cross walls; basal heterocyst globose, intercalary heterocyst quadrate; sheath distinct, thick, colourless and lamellated, 7-8µm broad and 6-7µm long. (Fig. 1 b, d and f).
Growth characteristics, pigments and biochemical attribute
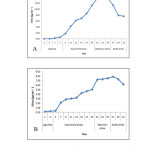
The growth curve analysis for both the strains in terms of chlorophyll a showed that the Microchaete strain with a longer exponential phase was found to be a slow- grower as compared to the Calothrix strain that has a longer lag phase (Fig. 2). The pigment analysis and biochemical attributes of the strains are shown in Table 1. Higher chlorophyll a (13.84 μg mL-1) and phycobiliproteins (103.35 mg g-1) content was found in Calothrix strain while the other Microchaete strain was higher in total carotenoid content (3.11 μg mL-1). The soluble protein concentration by Calothrix sp. isolated from Deodhar rice field was 79.80 μg mL-1and the lowest protein content was found in Microchaete sp.(42.47 μg mL-1). The total carbohydrate content accumulated by Microchaete sp. (115.71 μg mL-1) was higher whereas Calothrix sp. showed only 50.14 μg mL-1.
 |
|
 |
|
Molecular and phylogenetic analysis
A partial 16S rRNA gene sequences was obtained for both the strains and compared with closely related other nostocalean members comprising of Calothrix, Microchaete and Tolypothrix species from India and other parts of the world retrieved from the GenBank database. The Neighbor-Joining method was utilized to infer the evolutionary history. A phylogenetic tree reflecting the relationship among the strains and the related sequences of various nostocales strains has been presented in Fig. 3. The optimal tree with the sum of branch length = 0.14439346 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The evolutionary distances were computed using the p-distance method and are in the units of the number of base differences per site. Codon positions included were 1st+2nd+3rd+Noncoding. All positions containing gaps and missing data were eliminated resulting in a total of 567 positions in the final dataset.
 |
|
In the phylogenetic tree, Calothrix sp. (AUS-JR/MT/NT-036) was most closely related to the sequences of Tolypothrix sp. PCC 7504 from Mexico and Calothrix brevissima from Japan positioned in a large clade with other Calothrix species. This is congruent with recent molecular studies which place the genus in the clade with Tolypothrix and other closely related genera.27 However, the Microchaete sp. (AUS-JR/MT/NT-037) formed a robust clade with other Calothrix species and Tolypothrix species from Japan and is closely related Microchaete diplosiphon CCALA 811 from Mexico with high boostrap values.
Table 1: Pigment analysis and biochemical attributes (mean± SD) of the studied strains
|
Sl No |
Biochemical parameters |
|
Calothrix sp. (AUS-JR/MT/NT-036) |
Microchaete sp. (AUS-JR/MT/NT-037) |
|
1 |
Chlorophyll a (μg mL-1) |
13.84±0.08 |
7.87±0.02 |
|
|
2 |
Total carotenoid content (μg mL-1) |
1.12±0.003 |
3.11±0.34 |
|
|
3 |
Phycobiliproteins (mg g-1) |
Phycoerythrin (PE) |
8.57±0.052 |
3.11±0.052 |
|
Phycocyanin (PC) |
46.68±0.14 |
23.41±0.239 |
||
|
Allophycocyanin (APC) |
48.10±0.153 |
23.95±0.256 |
||
|
Total phycobiliproteins (mg g-1) |
103.35 |
50.47 |
||
|
4 |
Total carbohydrate (μg mL-1) |
50.14±0.08 |
115.71±0.08 |
|
|
5 |
Soluble protein(μg mL-1) |
79.80±0.20 |
42.47±0.31 |
Nucleotide accession numbers
The sequences obtained were submitted to NCBI with the respective accession numbers Calothrix sp. (AUS-JR/MT/NT-036) - KM252910 and Microchaete sp. (AUS-JR/MT/NT-037) - KM252911.
Systematic account on blue green algae occurring as biological soil crust from different regions of India were studied and detailed morphological study of Calothrix species were studied.28 The present study revealed that the isolated Calothrix strain showed high chlorophyll a accumulation and soluble protein content, unlike the Calothrix species isolated from Manwar and Pokharan region, Rajasthan that, these were poor in terms of chlorophyll and soluble protein content although showed remarkable potential for nitrogen fixation .29 The dissimilarity may be due to the difference in the habitat from where they have been isolated. The variation in the range of chlorophyll content from 11.16 μg mL-1 to 3.51 μg mL-1 amongst cyanobacterial strains of Calothrix isolated from different geographical locations of India was also reported.30 In another report, polyphasic characterization of Nostoc commune strains isolated from rice growing agroecosystems was also studied.31 Similar to the findings of Berrendero et al., 20086 where the genera Rivularia and Calothrix from GenBank database were intermixed in the phylogenetic inferences, our study showed that representatives of the genera Calothrix, Microchaete and Tolypothrix were intermixed hence showing great genetic divergence.6 Although 16S ribosomal gene analysis has been a method of choice for deducing phylogenies and establishing evolutionary relationships, morphological data have constantly been applied together with molecular data to obtain meaningful inferences concerning a more precise determination of the taxonomic status of cyanobacteria.32-33 Thus, the polyphasic approach used in the present study involving morphological and molecular procedures for investigating the genetic diversity and ecological significance amongst the isolates of Calothrix and Microchaete proved to be powerful and helpful.
Acknowledgement
The authors are thankful to the Department of Science and Technology, New Delhi, Government of India under the project fund (SERB/SR/SO/PS/96/2010) for providing financial assistance. The facilities required for carrying out this work provided by the Department of Biotechnology, Government of India during the research work is gratefully acknowledged.
References
- Prasanna, R., Jaiswal, P., Nayak, S., Sood, A. & Kaushik, B.D., Cyanobacterial diversity in the rhizosphere of rice and its ecological significance. Indian Journal of Microbiology, 49:89–97(2009).CrossRef
- Roger, P.A., Zimmerman, W.J. & Lumpkin, T.A., Microbiological management of wetland rice fields. F. B. Meeting Jr. (ed.), Soil microbial ecology. Application in agricultural and environmental management, M. Dekker, New York, 417-455 (1993).
- Nayak, S., Prasanna, R., Prasanna, B. M. and Sahoo, D. B., Analysing diversity among Indian isolates of Anabaena (Nostocales, Cyanophyta) using morphological, physiological and biochemical characters. World Journal of Microbiology and Biotechnology, 23: 1575–1584 (2007).
- Geitler, L., Cyanophyceae. Koeltz Scientific Books for Edition Karin Koeltz. Germany (1931).
- Komarek, J. & Anagnostidis, K., Modern approach to the classification system of cyanophytes. 4-Nostocales. Archives fur Hydrobiologie Supplement 82(3). Algological Studies, 56: 247-345 (1989).
- Berrendero, E., Perona, E. & Mateo, P., Genetic and morphological characterization of Rivularia and Calothrix (Nostocales, Cyanobacteria) from running water. International Journal of Systematic and Evolutionary Microbiology, 58:447-460 (2008).
CrossRef - Whitton, B.A. & Potts, M., Introduction to the cyanobacteria. In: Whitton, B.A. & Potts M. (eds.). The Ecology of Cyanobacteria. Kluwer Academic Press, Dordrecht, 1–11(2000).
- Uher, B., Morphological characterization of three subaerial Calothrix species (Nostocales, Cyanobacteria). Fottea, 7(1):33-38 (2007).
CrossRef - Rinkel, B.E., Calothrix- an evaluation of freshwater species in United States rivers and streams, their distribution and preliminary ecological findings. Proceedings of the academy of natural sciences of Philadelphia, 163: 43-59 (2014).
- Rouf, A.J.M.A., Phang, S.M. & Ambak, M.A., Depth distribution and ecological preferences of periphytic algae in Kenyir Lake, the largest tropical reservoir of Malaysia. Chinese Journal of Oceanology and Limnology, 28:856–867(2010).
- Yunes, J.S., Silveira, A.G., Suzuki, M.T., Camargo, M.G. and Werner,V.R., Diazotrophic growth and nitrogenase activity of cyanobacteria from the Patos Lagoon Estuary. Vittalle Rio Grande, 6:25–36(1994).
- Safiarian, M.S., Faramarzi, M. A., Amini, M., Soltani, N., Tabatabaei-Sameni, M. & Hasan-Beikdashti, M., Microalgal transformation of progesterone by the terrestrial-isolated cyanobacterium Microchaete tenera. Journal of Applied Phycology, 24(4):777-781(2012).
- John D.M., Whitton, B.A. & Brook, A. J., The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae: Second Edition Cambridge University Press (2011).
- Lukesova, A., Soil algae in four secondary successional stages on abandoned fields. Algological studies, 71:81-102 (1993).
- Rippka, R., Deruelles, J., Waterbury, J.B., Herdman, M. & Stanier, R.Y., Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Journal of General Microbiology, 111: 1–61(1979).
- Desikachary, T.V., Cyanophyta. I.C.A.R. Monograph on Algae. Indian Council of Agricultural Research, New Delhi (1959).
- Parsons, T.R. & Strickland, J.D.H., Determination of phytoplankton pigments. Journal of the Fisheries Research Board of Canada, 18: 117–127 (1965).
- Herbert, D., Philipps, P.J. & Strange, R.E., Chemical analysis of microbial cells. In: Norris, J.R. & Ribbons, D.W. (eds.) Methods in Microbiology. Vol. VB. Academic Press, New York, 209–344(1971).
- Spiro, R.G., Analysis of sugars found in glycoproteins. Methods in Enzymology, 8: 3–26 (1966).
CrossRef - Bennet, A. & Bogorad, L., Complementary chromatic adaptation in a filamentous blue green alga. The Journal of Cell Biology, 58: 419–435 (1973).
CrossRef - Parsons, T.R., Maita, Y. & Lalli, C.M., A manual of chemical and biological methods for seawater analysis. Pergamon press,Oxford, 73 pp. (1984).
- Smoker, J.A. & Barnum, S.R., Rapid small – scale DNA isolation from filamentous cyanobacteria. FEMS Microbiology letters, 56: 119–122 (1988).
- Nübel, U., Garcia-Pichel, F. & Muyzer, G., PCR primers to amplify 16S rRNA genes from cyanobacteria. Applied and Environmental Microbiology, 63: 3327–3332 (1997).
- Tamura K., Stecher G., Peterson, D., Filipski, A., & Kumar, S., MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution, 30: 2725-2729 (2013).
- Saitou, N. & Nei, M., The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4:406-425 (1987).
- Felsenstein, J., Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39:783-791 (1985).
CrossRef - Sanchez-Baracaldo, Hayes, P. K. & Blank, C. E., Morphological and habitat evolution in the cyanobacteria using a compartmentalization approach. Geobiology, 3:145-165 (2005).
- Tirkey, J. & Adhikary, S.P., Blue green algae in the biological soil crusts of different regions of India. Feddes Repertorium 117: 3-4, 280-306 (2006).
- Tiwari, O.N., Singh, B.V., Mishra, U., Singh, A. K., Dhar D. W. & Singh P.K., Distribution and physiological characterization of cyanobacteria isolated from arid zones of Rajasthan. Tropical Ecology, 46(2): 165-171(2005).
- Shalini, Dhar, D.W. & Gupta, R.K., Phylogenetic analysis of cyanobacterial strains of genus-Calothrix by single and multiplex randomly amplified polymorphic DNA-PCR World Journal of Microbiology and Biotechnology, 24:927–935(2008).
CrossRef - Borah, D., Rout, J. & Thajuddin, N., Polyphasic characterization of Nostoc commune (Cyanobacteria, Nostocaceae) isolated from rice growing agro-ecosystems of Dima Hasao district of Assam, North-East India. Phytotaxa, 161 (2): 111-120 (2014).
- Lyra, C., Suomalainen, S., Gugger, M., Vezie, C., Sundman, P., Paulin, L., & Sivonen, K., Molecular Characterization of Planktic Cyanobacteria of Anabaena, Aphanizomenon, Microcystis and Planktothrix International Journal of Systematic and Evolutionary Microbiology, 51:513–526 (2001).
CrossRef - Singh, S., Dhar, D. W. & Gupta, R. K., Morphological and molecular characterization of Calothrix isolates obtained from diverse environments in India. Microbiology, 80(3): 411-419(2011).
CrossRef







