Decomposition and Nitrogen Dynamics of tree Pruned Biomass Under Albizia Procera Based Agroforestry System in Semi arid Region of Bundelkhand, India
Garima Gupta 1 * , R.S Yadav 2 and Deepak Maurya 3
1
1,
Maya Collage of Agriculture and Technology,
Dehradun,
Uttarakhand
India
2
Indian Institute of Soil and Water Conservation,
RC-Datia,
MP
India
3
National Bureau of Soil Survey and Land Use Planning,
RC-Kolkata,
West Bengal
India
Corresponding author Email: garima811@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.12.3.24
Copy the following to cite this article:
Gupta G, Yadav R. S, Maurya D. Decomposition and Nitrogen Dynamics of tree Pruned Biomass Under Albizia Procera Based Agroforestry System in Semi arid Region of Bundelkhand, India. Curr World Environ 2017;12(3). DOI:http://dx.doi.org/10.12944/CWE.12.3.24
Copy the following to cite this URL:
Gupta G, Yadav R. S, Maurya D. Decomposition and Nitrogen Dynamics of tree Pruned Biomass Under Albizia Procera Based Agroforestry System in Semi arid Region of Bundelkhand, India. Curr World Environ 2017;12(3). Available from: http://www.cwejournal.org?p=1039/
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2017-07-17 |
|---|---|
| Accepted: | 2017-10-18 |
Introduction
Agroforestry practices are recommended to achieve soil conservation and it is a sustainable option in improving the livelihood as well as creating opportunities for the rural people of semi arid region of bundelkhand1. Most of the tree species recommended are fast-growing, leguminous species which can be pruned to a desirable height at appropriate frequencies. These pruning which consist of leaves and immature stems are added to the soil between crop rows. Therefore, while acting as a physical barrier to trap eroding soil, the trees also act as a source of mulch material to the soil. Gradual decomposition and nutrient release from added pruning could enhance the organic matter and nutrient status of the soil2,3 and influence the yield of the associated agricultural crop4,5. Tree species used in agroforestry differ in their ability to enhance soil fertility through addition of pruning6,7. This is because of the inter-species variation in biomass of pruning produced per year2, their nutrient contents8 and the rates of decomposition and nutrient release7,9. The rates of decomposition and nutrient release from added pruning are determined by the climatic factors such as rainfall and temperature regimes9,10&11 and by litter quality as determined by its lignin, polyphenol and nitrogen contents5,11. Biomass decomposition and nutrient release play an important part in selection of tree species for agroforestry because of the need to regulate the pattern of nutrient release and synchronize it with the nutrient demand of the associated agricultural crop2,12.
Albizia procera is the native and most common agrisilvicultural /agrosilvopastutal tree species of Semi arid regions of Central India. Being a fast growing legume species and having an immense potential for introduction in different types of soils and climatic conditions, it is planted in various states by the Forest Departments and also by farmers under Agro-forestry programmes. It fixes nitrogen through symbiotic bacteria present in root nodules and thus enhances and soil fertility. The present investigation aimed to analyze the impact of Albizia procera based agroforestry on improving soil organic carbon status and nitrogen availability.
Therefore, the objective of this experiment was to characterize and compare the patterns and amounts of biomass decomposition and nitrogen loss from Albizia procera in different land use in the semi arid region of Central India.
Materials and Methods
Study site and Plant Material
The study was conducted in six year old A. procera based agroforestry system at research farm of National Research Centre for Agroforestry, Jhansi, Uttar Predesh, India. The experimental field is situated at 250 27’ North latitude and 780 35’ East longitudes, 271 m asl in the semi arid region of the Central Indian Plateau. Average annual rainfall of the region is 806 mm, about 80 % of which occurs between June to September with intermittent dry spells. The mean monthly temperature is generally high, with high degree of variation between a maximum 39.80 C in May and June and minimum temperature of 5.80 C in December and January. In summer, temperature occasionally reached up to 480 C. The mean monthly evaporation in the region is highest in April- June (9.40-15.2mm) and it ranges from 1.90-6.00 mm during other months of the year. The soil in the experimental field is Parwa representing inter-mixed black and red soil group of bundelkhand region (U.P.), India, falling under the soil order Alfisol. It is medium in texture, moisture retentive and workability, prone to crust whenever drought spell exceeds 2-3 weeks even under mild evaporation situation.
The experiment field was established as agri-silviculture (crop + tree) system in July, 2000 with Albizia procera as the tree component. A. procera was planted in at spacing of 8m x 4m in plot size of 576 m-2 (18 trees plot-1) with three replications. Under A. procera blackgram – mustard crop sequence were taken as intercrop. In Kharif season (Black gram) the trails were fertilized with 20kg /ha N, 40 Kg/ha P and in rabi season (mustered) 60 Kg/ha N, 40 Kg/ha P and 40 Kg/ha K were applied. Inter crop black gram is rainfed in both pruning regime, therefore mustard is irrigated twice a year (1st at flowering and 2nd at siliquae formation).
Biomass Decomposition and Nutrient Analysis
Fresh pruned biomass (leaves, petiole and pods) of Albizia procera was collected from field and oven dried at 720C till constant weight. The standard litterbag technique14 was employed for characterizing litter decomposition dynamics.
Samples of 5.0 g of each component of the tree were transferred to nylon mesh bags (20x20 cm, 2 mm mesh size). The bags [270(3x3x5x6)] were randomly kept on the soil surface below respective tree canopies in experimental field. Each month, 5 bags for each biomass component of A. procera were collected from the floor of the different land sues. The biomass samples thus drawn were washed under a fine jet of water using a fine mesh screen to remove all the adhered soil particles, dried at 720 C to constant weight, weighed and ground in a Wiley Mill to pass through a 1mm mesh screen. Samples were analyzed for N analysis.
Data Analysis
To evaluate nutrient release pattern, nutrient remaining in the decomposing biomass were estimated by equation15
% Nutrient remaining = (C/C0) x (DM/DM0) x 102
Where,
C = Concentration of nutrient element in decomposition litter at the time of sampling
C0 = Concentration of nutrient element at the beginning of the study
DM = Mass of dry matter at the time of sampling
DM0 = Initial dry matter of the biomass kept for decomposition
The decay rate coefficient (k) of the decomposing pruned biomass of different component for the entire study period was calculated through the negative exponential decay model16 as represented by the equation:
X / X0 = e-kt
Further, following Olson (1963), the time required for 50 (half life) % weight losses was estimated from k values using the equation:
t50 = In (0.5) / -k = -0.693 / -k
Similarly, time taken for 95% decay can be estimated as follows
t 0.95=2.9957/k
The effect of land use of Albizia procera on decomposition, nutrient dynamics and cumulative impact on soil properties was tested by means of ANOVA using the General Linear Model of SYSTAT Ver.9 (SYSTAT Inc. 1998).
Results
Decomposition and decomposition coefficient
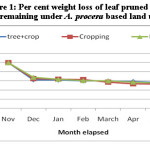
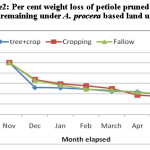
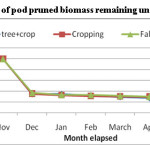
Average weight loss pattern in decomposing different components of pruned biomass of A. procera is shown in fig 1, 2 and 3. Weight loss pattern in six months under different land uses followed the trend: cropping > Fallow > A. procera + cropping for leaves; Cropping > A. procera + cropping > Fallow for the petiole and A. procera + cropping > Cropping > Fallow for pod. Data is further showen (Table 1) that for 95 per cent decay, pruned biomass leaves, petiole and pod is to took 568, 767 and 831 days; 969, 1046 and 1094 days; 414, 432 and 445 days, respectively, correspondingly under A. procera + cropping, cropping and fallow.
Table 1: Decomposition parameters of different components of pruned biomass of Albizia procera
|
Decay parameters |
Land uses |
||
|
A. procera + crop |
Cropping |
Fallow |
|
|
Leaves |
|||
|
Decay constant |
0.0046 |
0.0058 |
0.0050 |
|
t50 (days) |
131 |
177 |
192 |
|
t95 (days) |
568 |
767 |
831 |
|
t99 (days) |
949 |
1277 |
1385 |
|
Petiole |
|||
|
Decay constant |
0.0031 |
0.0034 |
0.0033 |
|
t50 (days) |
223 |
242 |
253 |
|
t95 (days) |
969 |
1046 |
1094 |
|
t99 (days) |
1616 |
1744 |
1823 |
|
Pod |
|||
|
Decay constant |
0.0071 |
0.0070 |
0.0068 |
|
t50 (days) |
96 |
100 |
103 |
|
t95 (days) |
414 |
432 |
445 |
|
t99 (days) |
690 |
720 |
741 |
Pearson correlation coefficient between pruned biomass substrate quality and decomposition coefficients
Interpretation of data (Table 2) revealed that decay rate coefficients were significantly and positively correlated with hemicellulose, N and P concentration of pruned leaves, petiole and pod with strong correlation in pod (r = 0.780). It is further interpretive that decay rate coefficients were significantly and negatively correlated with lignin, lignin/N, C/N, C/P and ADF of pruned leaves, petiole and pod with strong correlation in pod.
Table 2: Pearson correlation coefficient between substrate quality (Pruned biomass) and decomposition constant (k) averaged across the different land use
|
Quality parameter |
Leaves K |
Petiole K |
Pods K |
|
C |
-0.370* |
0.183 |
0.341 |
|
N |
0.596** |
0.598** |
0.780** |
|
P |
0.531** |
0.178 |
0.646** |
|
Lignin |
-0.622** |
-0.617** |
-0.724** |
|
C/N |
-0.593** |
-0.602** |
0.747** |
|
Lignin/N |
-0.614** |
-0.605** |
-0.757** |
|
ADF |
-0.551** |
-0.286 |
-0.647** |
|
Cellulose |
0.282 |
-0.218 |
-0.746** |
|
Hemi cellulose |
0.591** |
0.539** |
0.312** |
|
L/LC |
-0.594** |
-0.594** |
0.617** |
|
C/P |
-0.575** |
-0.103 |
-0.633 |
* Significant at P < 0.05, ** P < 0.01
Pearson correlation and linear regression between pruned biomass weight loss and environmental factors under A. procera based different land uses.
The effect of climatic factors on the decomposition rate was evaluated by correlating percent weight loss of pruned biomass (Table 3). Percent weight loss of leaf pruned biomass was positively correlated with air temperature and was significant (P<0.05) only in A. procera unpruned + crop. However, the soil temperature and soil moisture was positive and significantly (P<0.01) correlated with percent weight loss of leaf pruned biomass respectively in all land uses. Percent weight loss of petiole showed positive and significant (p<0.01) correlation with soil moisture and soil temperature in all land uses, while air temperature was significant only in A. procera unpruned + crop. Furthermore, soil moisture and soil temperature were also significantly (P<0.01) correlated with percent weight loss of pod, in all land uses.
Linear regression for the significant effect of climatic factors on percent weight loss (Table 4) was analyzed. The air temperature, soil moisture and soil temperature were found significantly (P<0.05) related with percent weight loss of all component in all land uses during decomposition.
Table 3: Pearson correlation coefficients between pruned biomass weight loss and environmental factors under A. procera based different land uses.
|
Environmental factors |
Pearson Correlation |
||
|
Leaves |
Petiole |
Pod |
|
|
A. procera unpruned + crop |
|||
|
Air Temperature |
0.76* |
0.76* |
0.76* |
|
Soil Moisture |
0.94** |
0.92** |
0.94** |
|
Soil Temperature |
0.90** |
0.89** |
0.91** |
|
Cropping |
|||
|
Air Temperature |
0.75 |
0.75 |
0.77* |
|
Soil Moisture |
0.93** |
0.94** |
0.93** |
|
Soil Temperature |
0.90** |
0.90** |
0.91** |
|
Fallow |
|||
|
Air Temperature |
0.70 |
0.75 |
0.73 |
|
Soil Moisture |
0.94** |
0.94** |
0.94** |
|
Soil Temperature |
0.87* |
0.90** |
0.88** |
* Significant at P < 0.05, ** P < 0.01
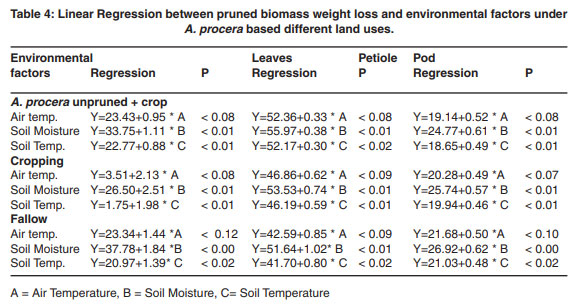 |
Table 4: Linear Regression between pruned biomass weight loss and environmental factors under A. procera based different and uses. click here to view table |
 |
|
 |
|
 |
|
Nitrogen Release
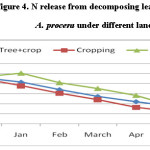
Result shown for N release from decomposing pruned biomass indicate that N content during decomposition increased initially followed by decrease. Figure 4 shows that leaf pruned biomass under cropping released higher N during decomposition followed under A. procera + crop and fallow. Petiole decomposition under cropping released 80.6 % N and was in the order: cropping > A. procera unpruned + crop (74.7 %) > fallow (64.1%). N content in decomposing pod pruned biomass increased initially. A. procera + crop, cropping, fallow, respectively. The corresponding N release from decomposing A. procera pod pruned biomass was 90.3, 93.7 and 90.6 percent.
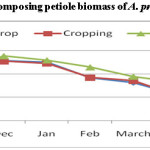
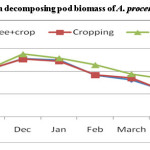
Figure 5 shows that N release from decomposing A. procera petiole pruned biomass differed widely among different land uses. Data showed that irrespective of different pruning and land use, N content in decomposing petiole pruned biomass increased initially and decreased finally. The final N content in decomposing petiole pruned biomass under A. procera unpruned + crop, cropping and fallow was 0.456, 0.371 and 0.667 per cent, respectively. From N release data (figure-6) it is evident that A. procera pod pruned biomass released N at faster rate followed by leaf and petiole. Across component of decomposing biomass, A. procera pruned biomass under cropping released maximum N and minimum N release was under fallow.
 |
|
 |
|
 |
|
Discussion
Substrate (litter/biomass) quality, climate and quantity and quality of decomposer organisms are the primary determinants of any biomass decay rates17,18. In the present work, the differences in rates of decomposition of different components of litter and biomass of A. procera under different land uses could be related to differences in substrate (litter/biomass) quality (Table 2) and variations in micro environment beneath A. procera in cropping and fallow. The higher concentration of N and lower C/N ratio in the biomass of A. procera was probably responsible for its faster decomposition and lower concentration of N in the petiole of A. procera brought slower rate of decomposition. A positive effect of N concentration on decomposition was also reported by several workers19,20,21,22,23&24. In A. procera, higher rate of biomass decomposition in agroforestry systems than to fallow might be due to high fertility status in agroforestry systems as evident from results of this study and favorable microenvironment for microbial population. Earlier, Anderson and Swift25 have pointed out that soil of high fertility favour faster rate of decomposition. Conducive microclimate under agroforestry systems might have also played role in faster litter decay as reported by several workers7,26,27&28.
Acknowledgment
This research work was conducted at the form and all lab work was carried out in the lab of National Research Centre for Agroforestry, Jhansi. I am very thankful to Director NRC-AF, Jhansi for his kind support and mentorship. I am to express out my appreciation to the Dr. R. S. Yadav, my PhD supervisor for sharing his pearls of wisdom with us during the course of this research. I am also thankful to our colleagues who directly or indirectly involved in form or lab work and provided expertise that greatly assisted this research work.
References
- Rai, A.K. (2008). Integrated resource management: an approach for sustainable livelihood. Development Alternative News Letter, 18(9).
- Young A (1989) Agroforestry for Soil Conservation. CAB International, Wallingford, UK, 274 pp
- Santa Regina I. (2001). Litterfall, decomposition and nutrient release in three semi-arid forests of the Duero basin, Spain. Forestry, 74: 347–358.
- Wilson GF, Kang BT and Mulongoy K (1986) Alley cropping: trees as sources of green-manure and mulch in the tropics. Biological Agriculture and Horticulture 3: 251–267
- Mafongoya PL, Nair PKR and Dzowela BH (1997a) Multipurposes tree prunings as a source of nitrogen to maize under semiarid conditions in Zimbabwe. 2. Nitrogen recovery rates and crop growth as influenced by mixtures and prunings. Agrofor Syst 35: 47–56
- Mafongaya PL, Giller KE and Palm CA (1998) Decomposition and nitrogen release patterns of tree prunings and litter. Agrofor Syst 38: 77–97
- Isaac SR, Nair MA. (2005). Biodegradation of leaf litter in the warm humid tropics of Kerala, India. Soil Biologyand Biochemistry, 37(9): 1656–1664.
- Palm CA (1995) Contribution of agroforestry trees to nutrient requirements in intercropped plants. Agrofor Sys 30: 105–124
- Mugendi DN and Nair PKR (1997) Predicting the decomposition patterns of tree biomass in tropical highland microregions of Kenya. Agrofor Syst 35: 187–201
- Meentemeyer V (1995) Meteorologic control of litter decomposition: With an emphasis on tropical environments. In: Reddy MV (ed) Soil Organisms and Litter Decomposition in the Tropics, pp 153–182. Oxford & IBH, New Delhi, India
- Devi NB, Yadava PS. (2006). Seasonal dynamics in soil microbial biomass C, N and P in a mixed-oak forest ecosystem of Manipur, North-east India. Applied Soil Ecology, 31: 220–227.
- Palm CA and Sanchez PA (1991) Nitrogen release from the leaves of some tropical leguminous trees as affected by their lignin and polyphenol contents. Soil Biol Biochem 23: 83–88
- Swift MJ (ed) (1987) Tropical soil biology and fertility (TSBF). Interregional Research Planning Workshop. Special Issue 13, Biology International. IUBS, Paris, France, 68 pp
- Anderson JM, and Ingram JS (1993). Tropical soil biology and Fertility, A Handbook of Methods. CAB International, Wallingford. pp 45-49.
- Bockhelm, J.G.; Jepsen, E.A. and Heisey, D.M. (1991). Nutrient dynamics in decomposing leaf litter of four tree species on a sandy soil in North Western Wisconsin. Can. J. For. Res., 21: 803-812.
- Olson JS. (1963). Energy storage and the balance of producers and decomposers in ecological systems. Ecology, 44: 322–331.
- Swift, M.J.; Heal, O.W. and Anderson, J.M. (1979). Decomposition in terrestrial ecosystems. Blackwell Scientific, Oxford, Pp.372.
- Jonsson LM, Dighton J, Lussenhop J, Koide RT. (2006). The effect of mixing ground leaf litters to soil on the development of pitch pine ectomycorrhizal and soil arthropod communities in natural soil microcosm systems. Soil Biologyand Biochemistry, 38(1): 134–144.
- Melillo, J.M.; Aber, J.D. and Musatore, J.F. 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecol., 63: 621-626
- Pandey, U. and Singh, J.S. (1982). Leaf litter decomposition in an Oak conifer forest in Himalaya. The effect of climate and chemical composition. For., 55: 47-59.
- Sandhu, J.; Sinha, M. and Ambasht, R.S. (1990). Nitrogen release from decomposing litters of Leucaena leucophloea in the dry tropics. Soil Biol. Biochem., 22: 859-863.
- Das, D.K. and Chaturvedi, O.P. (2003). Litter quality effects and decomposition rates of forestry plantations. Ecol., 44: 259-262
- Parton W, Silver WL, Burke I, Grassens L, Harmon ME, Currie B, King J, Adair EC, Brandt L, Hart S, Fasth B. (2007). Global-scale similarities in nitrogen release patterns during long-term decomposition. Science, 315: 361
- Yadav, R.S.; Yadav, B.L. and Chhipa, B.R. (2008). Litter dynamics and soil properties under different tree species in a semi arid region of Rajasthan, India. Sys., 73: 1-12
- Anderson, J.M. and Swift, M.J. (1983). Decomposition in tropical forest. In: Tropical rain forest: Ecology and management (S.L. Satton, T.C. Whitmore and A.C. Chadwickand, eds.). Blackwell, Oxford, Pp. 287-309.
- Meentemeyer, V. (1978). Macroclimate and lignin control of decomposition rates. Ecol., 59: 465-472.
- Moore, A.M. (1986). Temperature and moisture dependence of decomposition rates of hardwood and coniferous leaf litter. Soil Biol. Biochem., 18: 427-435.
- Issac, S.R. and Nair, M. (2002). Litter decay, weight loss and N dynamics of Jack leaf litter on open and shaded sites. Indian J. Agrofor., 4: 35-39.







