Status and Scope of Conventional Morphometry and its Integration with Bar Coding in J and K Fisheries.
1
Department of Zoology,
Central university of Jammu,
Rahya Suchani, Samba,
Jammu and Kashmir
India
Corresponding author Email: Shvetambri.zool@cujammu.ac.in
DOI: http://dx.doi.org/10.12944/CWE.17.3.23
Copy the following to cite this article:
Chib. A, Jasrotia S. Effects. Status and Scope of Conventional Morphometry and its Integration with Bar Coding in J and K Fisheries. Curr World Environ 2022;17(3). DOI:http://dx.doi.org/10.12944/CWE.17.3.23
Copy the following to cite this URL:
Chib. A, Jasrotia S. Effects. Status and Scope of Conventional Morphometry and its Integration with Bar Coding in J and K Fisheries. Curr World Environ 2022;17(3).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2022-06-01 |
|---|---|
| Accepted: | 2022-09-21 |
| Reviewed by: | 
 Sachinkumar R. Patil
Sachinkumar R. Patil
|
| Second Review by: |

 Umar Tangke
Umar Tangke
|
| Final Approval by: | Dr. Martha Luciane Fischer |
Introduction
The ichthyofauna found in riverine ecosystems provides essential supplies. Therefore, an important strategy for future sustainable use and conservation management of both the species and aquatic ecosystems is knowing the Ichthyofaunal population structure. 1
Because it serves as the basis for all other life sciences, taxonomic clarity is a vital prerequisite. As sampling and identification are the initial stages, therefore it is the duty of a researcher to precisely identify a species for the purpose of conservation and sustainable use. In recent times, biodiversity research is under priority and new fish identification techniques have been developed, however, their practical application during fish identification is still in its infancy. The most popular, straightforward, economical, and historical approach of identifying fish habitats is morphometric characterization.2,3,4
Morphometric characterization
All life forms express their phenotypic characters as a result of their genetic constituents and various environmental influences. Morphometrics is basically a more or less interwoven set of statistical procedures used to analyze variability in size and shape measurements of organs and organisms. Morphometric characterization is an old traditional practice being used in fisheries science since 1980’s, therefore there is huge array of literature available to enhance pertinent knowledge regarding this.5
In order to identify a species of fish, morphological characteristics such as body shape, scale size and count, colour pattern, number and relative location of fins, type and number of fin rays, and numerous relative measures of body parts are analysed 6.The statistical analysis of various morphometric characters has helped in recognition of various fish samples 7.
The morphometric characterization involves the analysis of both measureable and countable characters i.e. meristic characters.8Even though the meristic characters provide some evidence for stock separation, morphometric characters provide the best statistical separation.9 Analysis of morphometric characters help not only in correct identification of fishes but also in analysis of migration pattern, stock discrimination, reproductive isolation and commercially important species.10
Although external morphological characteristics are usually used to identify fish species, there are many distinct fish species and their many developmental phases that make it challenging to do so.11
Fish habitat and its ecology plays a major role in influencing its morphometric characterization.
A.Resource specialization and ecological conditions of Habitat
Fish exhibit the phenomenon of morphological adaptation depending on the resources and ecological conditions of their habitat, the availability of food, the temperature etc. As a result, it is possible for the same fish raised under different ecological conditions to develop different phenotypic characteristics.12,13,14,15,16,17 hence there is a risk of misidentification if visual assessment is employed to identify fishes.18Convergent and divergent adaptations also affect the correct identification.19
B.Morphologically identical species
In certain cases using morphometry for species identification yields errornous results because of close resemblance between the morphometric characters, 20 sometimes lack of quality in original description can also lead to errornous results21.
C.Early life stage
Morphological identification of eggs and larval fishes is more difficult, as their morphometric characters are not fully developed.22,23Morphometric characters are also subject to ontogenic transformation leading to error as they change during the process of development.24
D.Sex of the fish
Although it has been seen that the sex does not significantly influence the morphometric or meristic characters25 but error in morphological identification also depends on the sex of the fish eg. female sharks are more prone to misidentification as compared to male sharks, also the error rate is inversely propotional to body length.26
E.Mislabelled fishes
Fishes identified by conventional methods being sold in market could be mislabeled,either intentionally to fetch higher prices or unintentionaly due to close resemblance between species, incorrect identification of edible fishes can lead to fluctuations in market prices, also some times the mislabeled fishes being sold in the market could be poisonous.27,28,29 Therefore correct identification of fishes is essential to prevent their mislabeling.30Also traditional methodology helps in identifying the live or dead fish in good form, but not applicabe for identification of processed or mixed samples.31
F.More intraspecific variations than interspecific variations
Even with whole fish specimens, morphometric characterization is occasionally not a good enough option because they can display either more intraspecific variations or minor interspecific variations. For example, it can be challenging to distinguish between the various Barbus species that live in the Iberian Peninsula based solely on external morphology.32
G.Lack of classical taxonomists and pertinent literature
Taxonomists provide crucial knowledge about ecosystem thereby providing the key information in life sciences. It has been estimated that about 6000-10000 taxonomists are working worldwide with only a few of them are from developing countries that inhabit most of the Earth’s biodiversity.33,34This limited taxonomic community's distribution of competence is similarly uneven; more than 80% of taxonomists are either close to or older than 50 years of age, many among which are not having much computer knowledge.35 Therefore not able to send or retrieve literature electronically, hence there is a gap in expertise, among ecologically and phylogenetically important taxon ,36 which has lead to taxonomic impediment.37,38
In India, not only do we lack an updated checklist of fishes, but also the identification keys which have not been updated after the work of Talwar and Jhingran (1991)39, KC Jayaram(2010)40and Sarma and Mankodi (2017).41Also the original descriptions are referred to forever, irrespective of the quality of the paper. Making descriptive taxonomic literature available online is still a major task to promote quality in taxonomy, the unavailability of which impacts the taxonomic process; and often leads to erroneous results and phylogenetic assumptions.36
Academics are currently researching and utilising cutting-edge identifying techniques as a result of these difficulties. The application of DNA technologies for fish identification as a potent substitute tool has overcome the limitations of morphology-based identification approaches and the lack of local fish taxonomists.19
DNA BARCODING
Paul Hebert (2003) created the idea of DNA barcoding as a molecular identification tool, and it is now a frequently used approach for species identification even by non-specialists.Cytochrome oxidase subunit 1(COI), a mitochondrial DNA gene utilised as a universal bio-identifying system for an animal, is typically used as a short, standardised nucleotide sequence of DNA for the identification of fish in the process of DNA barcoding 42 Near the 5'-end of the mitochondrial gene cytochrome c oxidase subunit 1(COI) is a 648 base pair segment known as the animal barcode region.
The idea behind this method is that even within the same species, some components of an organism's DNA would vary individually. Finding these components at the species level was the very first task for the scientists who created this method. Geographic isolation causes some populations to stop sharing genetic material, and over time, separate gene pools evolve. These sub-populations maintain morphological similarity but diverge genetically, making them unable to mate and create offspring. These species are known as cryptic species. Because of this speciation, the morphological study of these populations can become questionable as we can’t be exactly sure. Such situations can be easily dealt with the molecular characterization method of DNA barcoding.43,44 Thus, it has now become a widely accepted and essential method for proper identification of species on a molecular level.
WHY COI?
This method is employed because mitochondrial DNA has unique properties, such as maternal inheritance, a high copy number per cell, a lack of recombination, a lack of introns, and a greater nucleotide substitution rate, which cause variations between species to rapidly accumulate. Due to COI's low mutation rate compared to other mitochondrial genes in animals, which facilitates its recovery using polymerase chain reaction, it was also chosen as the barcode marker.45A comparative analysis of three mitochondrial genes i.e 16S rRNA , cytochrome b , and cytochrome oxidase subunit I (COI) revealed that cyt b and COI are appropriate for clear identification of fishes whereas the 16S rRNA fails to discriminate closely related fish species.46
Bar coding of fishes globally and in India:
DNA barcoding finds immense application and success in fisheries and furthers the results of conventional morphometry. It is now well established and practiced all across the world. The costs involved in performing the experiment were very expensive in the past but are declining with advancements in technology.47
It is commonly believed that taxonomy and barcoding compete with one another for financing, but in reality, entities other than those supporting taxonomic work fund barcoding programmes. Therefore, bar coding would not in any way compete with traditional taxonomy, and the money spent on bar coding is also used to collect and preserve specimens, which are crucial for taxonomy. Therefore, the DNA barcoding programme has the potential to significantly increase fresh financing for museums, herbaria, and individual taxonomy labs rather than reducing support for taxonomy.48
Many workers have successfully tested the methodology of barcoding in not only identifying the species but also in the discovery of new species, monitoring of fisheries quotas, correct identification of fisheries products in market, keeping a check on trade of endangered species and identification of cryptic species.49,50,51,52,53Molecular characterization also assists in confirming the absence or presence of a species in given area. 54
In India, many researchers examined Ichthyofaunal diversity using DNA barcoding as a molecular appliance both for marine as well as freshwater fishes.One of the earliest work done on bar coding of fishes in India was by Lakra et al. (2011)55 for validating the application of bar coding.
Meanwhile many other researchers working on barcoding have confirmed its role in correct identification of fishes56,57,58,59,60 and also that barcoding enhances the global data base for quick identification of fishes, validates the checklist of fish fauna of the area, identifies invasive species and helps in formulation of conservation strategies. 61,62
Jammu and Kashmir at a glance
Jammu & Kashmir, the northernmost state in India, is located between 32.17" and 36.58" north latitude and between 73.26" and 80.30" east to west longitude. Due to its uneven topography, the weather in Jammu & Kashmir varies drastically.
Jammu Region
Although the region is sufficiently far west compared to the region's regular 40 to 50 mm (1.6 to 2 inches) of rainfall per month between January and March, the southern regions around Jammu typically have a monsoonal climate. Jammu town can see monthly extremes of rainfall of up to 650 millimetres in August and July, while temperatures in the warmer seasons can exceed 104°F. By early October, conditions are cool and incredibly dry, with little rain and temperatures of about 29 °C (84 °F). By September end, rainfall decreases.
Kashmir region
The region of Kashmir is renowned for its meadows, lakes, and springs. The earliest records of the area reveal that there was once a sizable lake in the valley, which was encircled by snow-covered mountains. It is thought that Kashmir Valley was once affected by earthquakes that it split apart the mountain wall near Baramulla, letting the water from Satisar Lake pour out and leaving behind karewas, or lacustrine mud, on the mountain edges. For hundreds of millions of years, Kashmir Valley was submerged beneath the Tethya Sea, and the valley's present-day tall sedimentary rock hills were originally submerged in water. The circular but erratic Valley of Kashmir was created in this way. There are many bodies of water in this area, which has a temperate climate.63
Water bodies and fish fauna of Kashmir Region
Table 1: Showing list of lentic and lotic water bodies in Kashmir region
Water Bodies of Kashmir64 | ||
1Dal lake | 15 Sheikhsar | 29 Jhelum River |
2Anchar. | 16 Waskursar | 30 Neelum River |
3Hokersar | 17Manasbal Lake | 31 Lidder |
4Nambli Narkara. | 18Vethnar Lake | 32 Rambi |
5Wular. | 19Ratan sar | 33 Sind river |
6Ajas Wetlands. | 20Gaditar Lake | 34 Veshaw |
7Hygham. | 21Sheshnag Lake |
|
8Tarsar Lake | 22Marsar Lake |
|
9Mirgund | 23Haigam Jeel |
|
10Vishansar | 24 Krishansar |
|
11Satsar | 25Nundkol Lake |
|
12Nilnag Lake | 26 Gadsar |
|
13Kounsarnar | 27 Demansar |
|
14Didufnag Lake | 28 Gangbal lake |
|
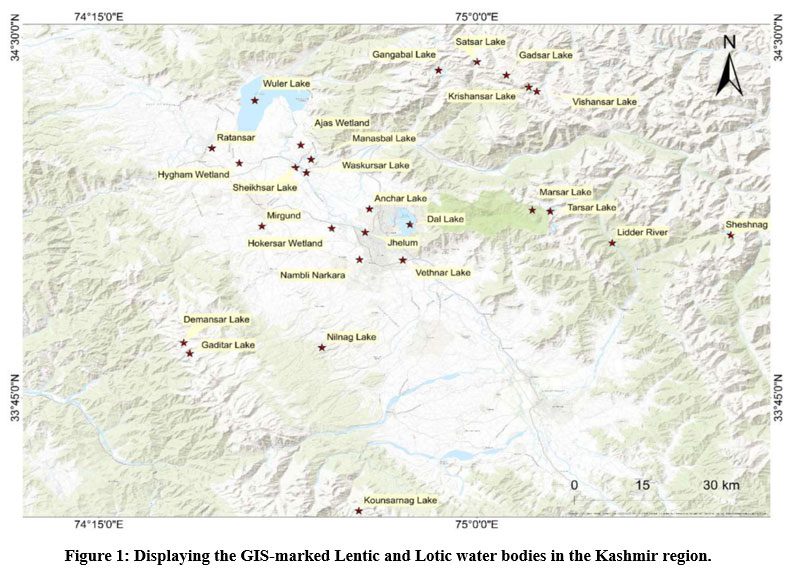 | Figure 1: Displaying the GIS-marked Lentic and Lotic water bodies in the Kashmir region.
|
Fish fauna of Kashmir region
The importance of the fish fauna has substantially increased since the endeavour of Haeckel in 1838,65 when he published “Fischeaus Caschmir” and thereafter various renowned ichthyologists have come up with very ingenious work like Day (1876)66, Silas (1960)67, Das and Subla (1964)68, Das and Nath (1965)69, Yousuf (1996),70 Kullander et al.(1999),71 Enderlin and Yousuf (1999),72 Balkhi (2007).73
Following table is based on compilation of most recent data obtained from details provided by numerous workers regarding the region’s fish fauna as determined by conventional morphometry.
Table 2: Status of fish fauna in Kashmir region.
WATERBODY/REFERENCE | Lotic | Lentic | FISHES FAUNA REPORTED |
1 River Jhelum a. khan and ali (2013)74 |
+ |
| ORDER:CYPRINIFORMES FAMILY:CYPRINIDAE 1 Schizothorax curvifrons 2 Schizothorax esocinus 3 Schizothorax plagiostomus 4 Schizothorax labiatus 5 Schizothorax niger 6 Cyprinus carpio |
b. Jan et al.( 2015)75 |
+ |
| ORDER:CYPRINIFORMES FAMILY:CYPRINIDAE 1 Schizothorax plagiostomus 3 Schizothorax esocinus ORDER:SALMONIFORMES FAMILY:SALMONIDAE 6 Salmo trutta fario 7 Salmo gairdneri |
c. Ahmed et al.(2017)76 |
+ |
| RIVER:JHELUM ORDER :CYPRINIFORMES FAMILY :CYPRINIDAE 1 Schizothorax Esocinus 2.Schizothorax Plagiostomus 3 Schizothorax Labiatus 4 Schizothorax Curvifrons 5.Schizothorax Niger 6 Cyrinus Carpio Communis 7.Cyprinus Carpio Specularis FAMILY:NEMACHEILIDAE 8 Crossochelius Diplochilus 9 Triplophysia Kashmirensis 10.Triplophysa Marmorata
|
2. Anchar Lake- Bashir et al. (2016)77 |
|
+ | ORDER :SALMONIFORMES FAMILY:SALMONIDAE 1 Salmo trutta fario ORDER:CYPRINIFORMES FAMILY:CYPRINIDAE 2 S. plagiostomus 3 Schizothorax ecocinus 4 S.labiatus 5 S. niger 6 S. richardsoni 7 S. curvifrons 8 Crossocheilus diaplochilus 9 Bangana diplostoma 10 Cypinus carpio communis 11 Cyprinus carpio specularis 12 Puntius conchonius 13 Carassius carassius FAMILY:NEMACHEILIDAE 14 Tritlophysa kashmirensis ORDER:CYPRINIDONTIFORMES FAMILY:POECILIIDAE 15 Gambusia affinis |
3. Wular Wetland- Brraich and malik (2016)78 |
|
+ | ORDER :CYPRINIFORMES FAMILY:CYPRINIDAE 1 Schizothorax labiatus 2 Schizothorax esocinus 3 Cyprinuscarpio var. communis 4 Schizothorax micropogon 5 Cyprinuscarpio var. specularis 6 Ctenopharyngodon idella 7 Schizothorax richardsonii 8 Schizothorax niger 9 Carassius carassius 10 Schizothorax curvifrons 11 Crossocheilus latius FAMILY:NEMACHEILIDAE 12Triplophysa marmorata |
b Wular Lake- Rumysa et al. (2016)79 |
|
+ | ORDER:CYPRINIFORMES FAMILY:CYPRINIDAE 1Cyprinus carpio specularis 2 Cyprinus carpio communis 3 Carassius carassius 4 Schizothorax niger 5 Schizothorax esocinus 6 Schizothorax curvifrons 7 Schizothorax labiatus 8 Schizothorax plagiostomus 9 Crossochelius diplochilus 10 Puntius conchonius FAMILY: COBITIDAE 11 Botia birdi FAMILY:NEMACHEILIDAE 12 Triplophysa kashmirensis 13 Triplophysa marmorata ORDER:CYPRINODONTIFORMES FAMILY:POECILIIDAE 14Gambusia affinis ORDER:SILURIFORMES FAMILY:SISORIDAE 15 Glyptothorax kashmirensis 16 Glyptothorax pectinoptrus |
c-Qadri et al.(2018)80 |
|
+ | ORDER:CYPRINIFORMES FAMILY:CYPRINIDAE 1 Cyprinus carpio var. communis 2Cyprinus carpio var. specularis 3Carassius carassius 4Schizothorax niger 5Schizothorax esocinus 6Schizothorax curvifrons 7Crossocheilus diplocheilus 8Puntius conchonius FAMILY:NEMACHEILIDAE 9Triplophysa spp
|
4.Dal lake -Ahmed et al.(2017)76 |
|
+ | ORDER :CYPRINIFORMES FAMILY :CYPRINIDAE 1Cyrinus Carpio Communis 2.Cyprinus Carpio Specularis 3 SchizothoraxCurvifrons 4. Schizothorax Niger 5. CrossocheilusDiplochilus 6. CarassiusCarassius ORDER:CYPRINODONTIFORMES FAMILY:POECILIIDAE 7. PuntiusConchonius 8. GambusiaHolbrooki FAMILY:BOTIDAE 9. BotiaBirdi
|
5. Hokersar Wetland- Mushtaq et al. (2019)81 |
|
+ | ORDER:CYPRINIFORMES FAMILY:CYPRINIDAE 1Cyprinus Carpio . Communis 2 Cyprinus Carpio Specularis 3 SchizothoraxNiger
|
6. River Viashaw- Hamid and Singh(2019)82 |
+ |
| ORDER :CYPRINIFORMES FAMILY:CYPRINIDAE 1 Schizothorax plagiostomas 2 Schizothorax curvifrons 3 Schizothorax esocinus 4 Schizothorax richardsonii 5 Triplophysa kashmirensis 6.Crossocheilus diplochilus |
b-Rashid and singh (2020)83 |
+ |
| ORDER:CYPRINIFORMES FAMILY:CYPRINIDAE 1 Schizothorax plagiostomus 2 Schizothorax labiatus 3 Schizothorax esocinus 4 Schizothorax curvifrons 5 Cyprinus carpio communis FAMILY:NEMACHEILIDAE 6 Triplophysa kashmirensis 7 Triplophysa marmorata ORDER: SILURIFORMES FAMILY: SISORIDAE 8 Glyptosternon reticulatum |
Table 3: Summary of fish species found in Kashmir region (based on compilation of data of table 2).
ORDER | FAMILY |
1.CYPRINIFORMES
| CYPRINIDAE 1 Bangana diplostoma |
COBITIDAE Botia birdi | |
NEMACHEILIDAE 1 BotiaBirdi | |
2.CYPRINIDONTIFORMES
| POECILIIDAE Gambusia affinis |
3.SALMONIFORMES
| SALMONIDAE 1 Salmo trutta fario 2 Salmo gairdneri |
NEMACHEILIDAE 1 Triplophysa kashmirensis 2 Triplophysa marmorata | |
4.SILURIFORMES
| SISORIDAE 1 Glyptothorax kashmirensis 2 Glyptothorax pectinoptrus 3 Glyptosternon reticulatum |
.jpg) | Figure 2: Percentage contribution of different orders to fish diversity of Kashmir region (according to table 3's data) .
|
Analysis of the data in table 2 demonstrates that only a small portion of the total number of water bodies existent (given in table 1) have been extensively studied, leaving the majority of water bodies undiscovered.
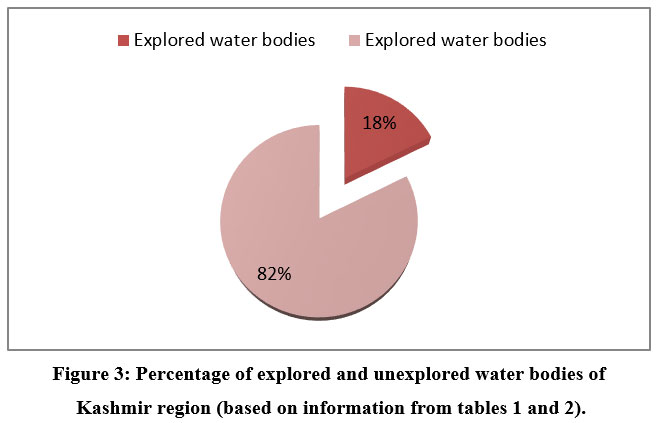 | Figure 3: Percentage of explored and unexplored water bodies of Kashmir region (based on information from tables 1 and 2).
|
Current Status of Barcoding integrated with conventional morphometry in the Valley.
Analysis of the data (table 2) reveals that different researchers have identified different species in the same body of water, and also that there is not a significant temporal gap between those reports, therefore taxonomic ambiguity must have been a major factor in the inconsistent results reported, as many of the species native to the region are challenging to identify using conventional morphometry. Like Identification of species of genus Schizothorax and Tryplophysa through conventional methodology can some times lead to errornous results.
The morphology of the genus Schizothorax is strikingly similar, making it challenging to distinguish between species based on morphological characteristics.Lately collaboration of barcoding with conventional morphometry has been adopted and tested. After performing morphometric characterization to see if barcoding can aid in accurate species identification in fishes, researchers DNA-barcoded schizothorax species from the Neelum and Jhelum rivers in Azad Kashmir. The results showed that barcoding is accurate, dependable, and has enormous potential for species identification.84 In addition to that a similar study on five different species of Schizothorax validated the role of cytochrome oxidase I in species delineation in conjunction with morphometric information and also that the Sequence-based phylogenetic analysis reveals different species groups85 ( Bashir et al., 2015).
This integrated approach was also used to characterise two significant fish species from the Kashmir valley, Triplophysa marmorata and T. kashmirensis. Due to the inadequate original descriptions and the dearth of positive reviews, it is difficult to distinguish between these two species. A morphometric and molecular analysis was carried out with this in mind. Investigation concluded that these two taxonomic Triplophysa taxa should be accepted as valid based on morphological and mtDNA COI sequence analyses. These findings can help ichthyologists better understand the ichthyofauna of the Kashmir valley and may aid them in developing methods for protecting and managing these lesser-studied native tiny species within their area of distribution21
Water bodies and fish fauna of Jammu Region
Jammu region with subtropical climate is blessed with a number of lentic and lotic water water bodies offering ample water resources for development of fisheries
Table 4: Showing list of lentic and lotic water bodies in Jammu region.
WATER BODIES OF JAMMU 64 | |
1.Gharana Wetland | 11.Chenab River |
2.Pargwal Wetland | 12.Tawi River |
3.Sangral Wetland | 13.Ravi River |
4.Nanga Wetland | 14.Poonch River |
5.Kukrian Wetland |
|
6.Cheshara |
|
7.Mansar Lake |
|
8.Surinsar Lake |
|
9.Thein |
|
10.Bahu |
|
Fish fauna of Jammu region
Icthyofauna of the Jammu region was intensively investigated for the first time by Das and Nath (1965,1966)86 eventually many workers have reported fish fauna from the region like Das and Nath (1971)87, Malhotra et al. (1975)88, Joshi et al. (1978)89,Tilak (1971)90 Dutta and Malhotra(1984)91, Jyoti et al. (2006)92 and Balkhi (2007).73
Following table is based on a collection of recent information on fish diversity provided by multiple workers for various lentic and lotic water bodies in the Jammu region, as determined by conventional morphometry.
Table 5: Status of fish fauna reported from the Jammu region
WATERBODY/ REFERENCE
| LOTIC | LENTIC | FISHES FOUND
|
1. River Chenab: a.Baba et al. (2014)93
|
+ |
| ORDER:CYPRINIFORMES FAMILY:CYPRINIDAE 1. Schizothorax plagiostomus FAMILY:NEMACHEILIDAE 19 Nemacheilus botia FAMILY:COBITIDAE 20.Botia dayi ORDER:SYNBRANCHIFORMES FAMILY:MASTACEMBELIDAE 21 Mastacembalus armatus 22 Macrognathus pancalus ORDER:SILURIFORMES FAMILY:SISORIDAE 23. Glyptothorax botium 24. G. pectinopterum 25. Glyptosternum maculatum 26 Bagarius bagarius FAMILY:SILURIDAE 27 Wallago attu FAMILY:BAGRIDAE 28. Mystus seenghala 39. M. bleekeri 30 Mystus cavasius ORDER:BELONIFORMES FAMILY:BELONIDAE 31 Xenentodon cancila
|
b.River Chenab Kishtwar district. Bhutyal and Langer(2015) 94 |
+ |
| ORDER:SALMONIFORMES FAMILY:SALMONIDAE. 1Oncorhynchus mykiss ORDER:SILURIFORMES FAMILY : SISORIDAE 2 Glyptosternum reticulatum ORDER :CYPRINIFORMES FAMILY:CYPRINIDAE 3 Schizothorax sp. |
2.River Tawi Gandotra et. al (2017)95 |
+
|
| ORDER :CYPRINIFORMES FAMILY:CYPRINIDAE 1 Garra gotyla ORDER :BELONIFORMES FAMILY:NEMACHEILIDAE 14 Schistura montanus ORDER:MASTACEMBELIFORMES FAMILY:MASTACEMBELIDAE 16 Mastacembelus pancalus ORDER:PERCIFORMES CHANNIDAE 18 Channa punctatus ORDER: SILURIFORMES SISORIDAE 20 Bagarius yarrelli BAGRIDAE 21 Mystus seenghala
|
3.River Basantar Sharma and Dutta (2012)96 |
+ |
| ORDER : OSTEOGLOSSIFORMES FAMILY : NOTOPTERIDAE 1Notopterus notopterus ORDER : CYPRINIFORMES FAMILY:CYPRINIDAE 2 Catla catla FAMILY:COBITIDAE 19. Botia dayi ORDER:SILURIFORMES FAMILY:BAGRIDAE 20. Mystus bleekeri FAMILY:SILURIDAE 24. Ompak bimaculatus FAMILY:SCHILBEIDAE 26.Clupisoma garua FAMILY:SISORIDAE 27. Bagarius bagarius ORDER : PERCIFORMES FAMILY : NANDIDAE 30. Badis badis FAMILY:CHANNIDAE 31. Channa punctatus ORDER : SYNBRACHIFORMES FAMILY: MASTACEMBELIDAE 34. Mastacembelus armatus
|
4.Ornamental Fishes of Jammu Region a.Vohra et al. (2013)97 |
+ |
+ | ORDER:CYPRINIFORMES FAMILY:CYPRINIDAE 1 Danio devario FAMILY:BOTIIDAE 10 Botia dayi FAMILY:NEMACHEILIDAE 11 Noemachilus botia FAMILY:COBITIDAE 12 Lepidocephalichthys guntea ORDER:SILURIFORMES FAMILY:BAGRIDAE 13 Mystus bleekri FAMILY:HETEROPNEUSTIDAE 14 Heteropneustes fossilis ORDER:SYNBRANCHIFORMES FAMILY:MASTACEMBELIDAE 15 Macrognathus aculeate ORDER:ANABANTIFORMES FAMILY:OSPHRONEMIDAE 19 Trichogaster fasciatus ORDER :BELONIFORMES FAMILY:BELONIDAE 20 Xenentodon cancilla |
ornamental fishes by b. Arif et al. (2019)98 |
+ |
+ | ORDER :CYPRINIFORMES FAMILY:CYPRINIDAE 1 Salmostoma bacaila FAMILY:NEMACHEILIDAE 19 Nemacheilus botia ORDER:SILURIFORMES FAMILY :BAGRIDAE 20 Mystus seenghala FAMILY:HETEROPNEUSTIDAE 22 Heteropneustes fossilis ORDER:BELONIFORMES FAMILY:BELONIDAE 23 Xenentodon cancilia ORDER:SYNBRACHIFORMES FAMILY:MASTACEMBELIDAE 24 Mastacembelus armatus ORDER:ANABANTIFORMES FAMILY :CHANNIDAE 26 Channa punctatus FAMILY:OSPHRONEMIDAE 29 Trichogaster fasciatus ORDER:PERCIFORMES FAMILY:BADIDAE 30 Badis badis
|
5.Erstwhile Udhampur District Dutta (2015)99
|
+ |
+ | ORDER : CYPRINIFORMES FAMILY : CYPRINIDAE 1 Hypothalmichthys molitrix 3 Aspidoparia morar 10 Danio devario 13 Amblypharyngodon. mola 15 Cyprinus carpio communis 16 C. carpio specularis 17 Tor. tor ORDER : SILURIFORMES FAMILY : BAGRIDAE 52. Mystus. bleekeri 55. Aorichthys. seenghala FAMILY :SILURIDAE 56. Ompok. bimaculatus 58. Wallago. attu FAMILY : SCHILBEIDAE 59. Eutropiichthys. vacha FAMILY : AMBLYCIPITIDAE 60. Amblyceps.mangois FAMILY :SISORIDAE 61. Bagarius. bagarius 62.Glytosternon.reticulatum 63. Glyptothorax. pectinopterus 64. G. indicus 65. G. telchitta telchitta 66. G. cavia 67. G. Kashmirensis 68. G. punjabensis FAMILY:HETEROPNEUSTIDAE 69. Heteropneustes. fossilis ORDER :BELONIFORMES FAMILY:BELONIDAE. 70. Xenentodon. cancila ORDER : SYNBRANCHIFORMES FAMILY: MASTACEMBELIDAE 71. Macroganthus. pancalus 72. Mastacembelus. armatus ORDER : PERCIFORMES FAMILY: BELONTIDAE 73. Colisa fasciatus FAMILY :CHANNIDAE 74. Channa. Orientalis 75. C. punctatus ORDER :SALMONIFORMES FAMILY : SALMONIDAE 76. Salmo trutta fario
|
6.River Ujh, an important tributary of the river Ravi. Rathore and Dutta (2015)100 |
+ |
| ORDER : CYPRINIFORMES FAMILY: CYPRINIDAE 1. Salmostoma bacaila FAMILY: BALITORIDAE 24. Acanthocobitis botia FAMILY:COBITIDAE 25. Botia almohare 26. Botia birdi 27. Lepidocephalichthys guntea ORDER : SILURIFORMES FAMILY : BAGRIDAE 28. Aorichthys seenghala 29 Mystus bleekeri 30 Mystus Vittatus FAMILY : SILURIDAE 31 Ompok bimaculatus 32 Wallago attu FAMILY:AMBLYCIPITIDAE 33. Amblyceps mangois FAMILY:SISORIDAE 34. Bagarius bagarius 35. Glyptothorax pectinopterus 36. G. stoliczkae 37. G. telechitta telechitta ORDER:BELONIFORMES FAMILY:BELONIDAE 38. Xenentodon cancilia ORDER:SYNBRANCHIFORMES FAMILY:MASTACEMBELIDAE 39.Mastacembelus armatus 40.Macrognathus pancalus ORDER:PERCIFORMES FAMILY:CHANNOIDAE 41. Channa punctatus 42. C. orientalis |
7.Chadwal Stream Khajuria et al.( 2015)101
|
+ |
| ORDER :CYPRINIFORMES FAMILY:CYPRINIDAE 1 Labeo boga 3 Labeo dero 4 Labeo calbasu 5 Labeo rohita 6 Danio devario 7 Crossocheilus latius 8 Puntius sophore FAMILY:NEMACHEILIDAE 14 Nemacheilus botia ORDER :BELONIFORMES FAMILY:BELONIDAE 15 Xenentodon cancila ORDER:ANABANTIFORMES FAMILY:CHANNIDAE 16Channa punctatus |
8. Sunderbani(stream) Gandotra and Sharma (2015)102 |
+ |
| ORDER:CYPRINIFORMES FAMILY: CYPRINIDAE 1 Schizothorax richardsonii 9 Puntius sophore ORDER: SILURIFORMES FAMILY :SISORDAE 13 Glyptothorax pectinopterus |
9. Lotic waterbodies of r.s.pura Tehsil Kour et al. (2015)103 |
+ |
| ORDER: CYPRINIFORMES FAMILY:CYPRINIDAE 1 Amblypharyngodon mola 6. Puntius ticto FAMILY:COBITIDAE 15 Lepidocephalichthys guntea 16 Noemacheilus botia ORDER:SILURIFORMES FAMILY:BAGRIDAE 17. Mystus bleekeri 18. Mystus seenghala 19. Mystus vittatus FAMILY:SILURIDAE 20. Wallago attu FAMILY:SCHILBEIDAE 21. Pseudoeutropius athernioides ORDER:PERCIFORMES FAMILY:AMBASSIDAE 22. Ambassis nama ORDER :BELONIFORMES FAMILY:BELONIDAE 23. Xenentodon cancila ORDER :OPHIOCEPHALIFORMES FAMILY:OPHIOCEPHALIDAE 24. Channa punctatus ORDER:MASTACEMBELIFORMES FAMILY:MASTACEMBELIDAE 25. Mastacembelus pancalus |
10.Wajoo nullah( an important tributary of the river) Dutta (2016)104
|
+ |
| ORDER: OSTEOGLOSSIFORMES FAMILY:NOTOPTERIDAE 1 Notopterus notopterus 2 Chitala chitala ORDER: CLUPEFORMES FAMILY: CLUPEIDAE 3.Gudusia chapra ORDER :CYPRINIFORMES FAMILY:CYPRINIDAE 4 Securicula gora 28. L.calbasu FAMILY: BALITORIDAE 37. Acanthocobitis botia FAMILY: COBITIDAE 38. Botia almorhae 39. B. lohachata 40. Lepidocephalus guntea ORDER: SILURIFORMES FAMILY: BAGRIDAE 41. Rita rita 42. M.vittatus 43. M.bleekeri 44. M.cavasius 45. Aorichthys seenghala FAMILY: SILURIDAE 46.Ompok pabda 47. Wallago attu FAMILY: SCHILBIDAE 48. Pseudeutropius atherinoides FAMILY:AMBLYCPTIDAE 49 Amblyceps mangois FAMILY:SISORIDAE 50. Bagarius bagarius 51. Gagata cenia FAMILY:HETEROPNEUSTES 52 Heteropneustes fossilis ORDER : BELONIFORMES FAMILY: BELONIDAE 53. Xenontodon cancila ORDER :SYNBRANCHIFORMES FAMILY :MASTAMCEMBELIDAE 63. Macroganthus pancalus 64. Mastacembelus armatus
|
11.Tehsil mendhar (district- poonch) Hussain and Dutta (2016 )105 |
+ |
+ | ORDER : CYPRINIFORMES FAMILY : CYPRINIDAE 1 Cyprinus carpio communis 3 Ctenopharyngodon idellus 5 L. dero FAMILY :BALITORIDAE 14 Schistura prashadi FAMILY :COBITIDAE 17 Botia birdi ORDER : SILURIFORMES FAMILY : SISORIDAE 18 Glyptothorax punjabensis FAMILY : SILURIDAE 19 Ompok pabda ORDER : SYNBRANCHIFORMES FAMILY : MASTACEMBELIDAE 20 Mastacembelus armatus ORDER :PERCIFORMES FAMILY:CHANNIDAE 21 Channa orientalis |
12.Selum nullah and Aik nullah Khajuria et al.(2016)106 |
+ |
| ORDER :CYPRINIFORMES FAMILY:CYPRINIDAE 1 Puntius conchonius 4 Labeo boga 8 Crossocheilus latius FAMILY:COBITIDAE 11 Rasbora rasbora ORDER:SILURIFORMES FAMILY:SILURIDAE 15 Heteropnuestes fossilis FAMILY:BAGRIDAE 16 Mystus bleekeri FAMILY:SISORIDAE 17 Gagata gagata ORDER:SYNBRANCHIFORMES FAMILY:MASTACEMBALIDAE 18 Mastacembalus armatus ORDER :OPHIOCEPHALIFORMES FAMILY:OPHIOCEPHALIDAE 19 Channa punctatus ORDER:BELONIFORMES FAMILY:BELONIDAE 20 Xenentodon cancila
|
13. Ichthyofauna of Rajouri district. Nisa et al. (2020)107 |
+ |
+ | ORDER:CYPRINIFORMES. FAMILY:DANIONIDAE 1Barilius vagra FAMILY:NEMACHEILIDAE. 2 Triplophysa sp. FAMILY :CYPRINIDAE 3 Cirrhinus mrigala ORDER:SILURIFORMES. FAMILY:SISORIDAE 16 Glyptothorax pectinopterus |
14 River Ravi Dutta (2021)108 |
+
|
|
ORDER: OSTEOGLOSSIFORMES FAMILY: NOTOPTERIDAE 1. Notopterus notopterus 2. Chilata chitala ORDER: CLUPEIFORMES FAMILY: CLUPEIDAE 3. Gudusia chapra ORDER: CYPRINIFORMES FAMILY:CYPRINIDAE 4. Salmophasia bacaiIa 5. Salmophasia phulo 6. Salmophasia Punjabensis 7. Securicula gora 8. Asidoparia morar 9. Barilius vagra vagra 10. B. barila 12. B. radiolatus Gunther 13. B. bendelisis 14. Raiamas bola 17. Esomus danricus 27. P. conchonius 29. P. ticto 30. P. chola 44. Schizothorax. richardsonii FAMILY: BALITORIDAE 49. Nemacheilus corica 51. Schistura prashadi 52. S. montanus FAMILY: COBITIDIAE 54. Botia almorhae 55. Botia birdi 57. Lepidocephalus. guntea ORDER: SILURIFORMES FAMILY: BAGRIDAE 58. Rita rita 59. Mystus bleekeri 61. M. vittatus 63. Aorichthys seenghala 64. A. aor FAMILY: SILURIDAE 65. Ompok pabda 66. Wallago attu FAMILY: SCHILBIDAE 67. Ailia punctata 68. Neotropius atherinoides 69. Clupisoma garua 71. Eutropiichthys murius FAMILY: AMBLYCIPITIDAE 73. Amblyceps mangois FAMILY:SISORIDAE 74. Bagarius. bagarius 75. Gagata. cenia 76. Glyptosternum reticulatum 79. G. pectinopterus 81. G. telchitta FAMILY: CLARIIDAE 82. Heteropneustes fossilis 83. Clarius batrachus ORDER: SALMONIFORMES FAMILY: SALMONIDAE 84.Salmo trutta fario FAMILY: BELONIDAE 85. Xenentodon. cancila ORDER: SYNBRANCHIFORMES FAMILY: MASTACEMBELIDAE 86. Macroganthus aral 88. Mastacembelus armatus ORDER: PERCIFORMES FAMILY: CHANDIDAE 89. Chanda nama FAMILY: NANDIDAE 92. Nandus nandus FAMILY: GOBIIDAE 93. Glossogobius giuris FAMILY :CHANNIDAE 94. Channa marulius 96. C. punctatus 97. C. striatus
|
15.Fish Fauna of River Sewa Gupta and Dutta (2021)109
|
+ |
| ORDER:CYPRINIFORMES FAMILY:CYPRINIDAE 1 Barilius vagra ORDER:SILURIFORMES FAMILY:SISORIDAE 7 Glyptothorax stoliczkae ORDER:SALMONIFORMES FAMILY:SALMONIDAE 8 Salmo trutta fario
|
16.Mansar-Surinser Lake (Information Sheet on Ramsar sites) |
|
+ | ORDER:CHANNIFORMES FAMILY:OPHIOCEPHALIDAE 1.Channa gachua 2.Channa punctatus ORDER:CYPRINIFORMES FAMILY:CYPRINIDAE 3.Cyprinus carpio 4.Danio rerio 5.Labeo rohita 6.Puntius chonchonius 7.Rasbora rasbora FAMILY:BELONTIIDAE 8.Trichogaster fasciatus FAMILY:MASTACEMBELIDAE 9.Mastacembelus armatus
|
17.Gharana wetland (Information Sheet on Ramsar sites) |
|
+ | ORDER:CYPRINIFORMES FAMILY:CYPRINIDAE 1.Puntius sophore 2.Puntius ticto 3.Rasbora rasbora FAMILY:CHANNIDAE 4Channa marulius 5Channa orientalis 6Channa punctatus 7Channa striatus FAMILY:BELONTIIDAE 8Trichogaster fasciatus FAMILY:CLARIIDAE 9Heteropneustes fossilis |
According to an analysis of the data in table 5, only a small percentage of the total number of water bodies that are known to exist (as shown in table 4) have undergone comprehensive research, leaving the bulk of water bodies unexplored.
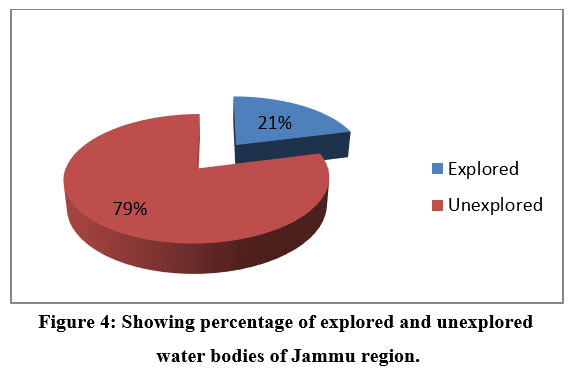 | Figure 4: Showing percentage of explored and unexplored water bodies of Jammu region.
|
Status of Bar Coding in Jammu Region
The present study, which is based on an examination of past findings, found that several lentic and lotic water bodies in the area has documented about 160 species. However, due to a lack of molecular characterization work, the employment of contemporary methodologies is still in its infancy.
Arif and Gandotra (2017)110 carried out DNA barcoding of ornamental fishes in various water bodies in the Jammu region for the first time, verifying its usage for precise species identification. Analysis of the economic value of the fish fauna of the region reveals that the majority are food fishes, with ornamental fishes accounting for the second-highest percentage, and the remainder have both food and ornamental fishes as economic status. Since food and forage fish make up the majority of the fish in the area, molecular characterization of the major section of fish diversity remains untouched, placing the data available regarding the current status of fish fauna under uncertainty.
As shown by the data in table 5, the Jammu region is blessed with a rich diversity of fish species, many of which are generally difficult to identify morphometrically. However, bar coding is still in its infancy in this area. Major factors contributing to this research gap include outdated knowledge of current techniques, lack of funding, greater expertise in conventional methodologies, and most importantly, the fact that basic research is being neglected in favour of applied research as taxonomy has taken a backseat over time. Because most of the species lack accurate taxonomic resolution, analyses of the historical record of fish distribution, making temporal comparisons, and tracking the proper phylogeny have all been impeded.
Discussion
Due to the difference in topography, larger number of lotic water bodies, and more favourable climatic conditions, the fish diversity in the Jammu region is greater than that in the Kashmir region. Cypriniformes is the most prevalent order in both Jammu and Kashmir due to their great level of adaptability and capacity to occupy any area.Along with the endemic species of the genus Schizothorax, many of the fishes listed above (tables 4 and 5) are exotic species, such as carps, which are not native to the area and were introduced by the state fisheries department.
Anthropogenic stress, declining fish diversity and need for conservational measures
Fish, which have a heterothermic body temperature, are easily impacted by changes in the physicochemical characteristics of the body of water they reside in. 111,112The aquatic ecosystem is being negatively impacted by climate change and anthropogenic factors such as pollution, overfishing, hydropower projects, etc. These factors also cause coral bleaching, the loss of coastal wetland, changes in the distribution and timing of freshwater flow, and a decline in fish diversity. 113
The largest freshwater lake in Asia, Wular Lake, is home to several fish species. However, eutrophication caused by human activity, which alters the water's physicochemical properties and impairs ecological conditions, has caused the extinction of numerous schizothorax species that are adapted to clean water.79,80Fish population of Schizothorax plagiostomus and Schizothorax esocinus in Dal lake has also been affected because of the constantly degrading water quality of the water body.114
The River Jhelum, a significant tributary of the Indus River System that drains through the entire state of Kashmir, is a celebrated river economically and a significant source of water for expanding human population and irrigation.However, the water body's shifting biological conditions have also encouraged the effective colonisation of foreign fish species with exceptional adaptability. The Viashaw River, a left tributary of the Indus River System, is also being affected by illicit mining and overfishing, which is reducing the diversity of fish. 83,114
Water pollution has affected the fish fauna of Jammu region as well, a comparative analysis of fish fauna of different water bodies have revealed a decline in fish diversity,96 especially those of threatened species 115.Therefore for conservation of the river system allochthonous sources of pollution like sewage, dumping of garbage, mining and agricultural activities needs to be monitored. Different conservation measures like using a species as flagship species, creating awareness and starting different projects towards conservation needs to be adopted, 116also small hydropower projects should be prioritised over large reservoir-based hydropower projects since they are more environmentally friendly and have fewer negative effects on flora and wildlife.117
Research Gap and Future Prespective
Only a small number of the area's waterbodies have been thoroughly examined; the remainder of the wetlands and many lentic water bodies have mostly remained uncharted due to accessibility concerns (remote location), financial limitations, and the locals' intense religious convictions. Fish production in the area can increase significantly when the existing resources (waterbodies and fish fauna) are used wisely in order to meet the demands of UT's growing population. As the majority of the water bodies in the Jammu and Kashmir region are unexplored, concealing a substantial portion of the fish flora and its gene pool, an integrative strategy can assist close the study gap.
Therefore a collaboration on the molecular aspect of fisheries in J and k especially in Jammu region with conventional taxonomy will immensely help in better understanding of the fish ecology of the region and will also aid in properly identifying and conserving the gene pool thereby boosting the growth of the economically important fishes belonging to this region.
Acknowledgments
The authors acknowledge the support and guidance received from the Department of Zoology, Central University of Jammu, J&K. Anchal Chib (NET-JRF) is also thankful to University Grants Commission for providing Junior Research Fellowship.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Paller VGV, Corpuz MNC, OcampoPP (2013). Diversity and Distribution of Freshwater Fish Assemblages in Tayabas River, Quezon (Philippines). Philippine Journal of Science 142 (1): 55-67.
- Cadrin SX, Silva VM (2005). Morphometric variation of yellowtail flounder. ICES Journal of Marine Science, 62(4), 683–694. doi:10.1016/j.icesjms.2005.02.006.
- Chaklader R, Bakar Siddik MA, Nahar A (2015) Taxonomic diversity of paradise threadfin polynemus paradiseus (Linnaeus, 1758) inhabiting southern coastal rivers in Bangladesh. Sains Malaysiana, 44(9), 1241–1248. doi:10.17576/jsm2015-4409-04.
- Siddik MAB, Hanif MA, Chaklader MR, Nahar A, Mahmud S (2015) Fishery biology of gangetic whiting Sillaginopsis panijus (Hamilton, 1822) endemic to Ganges delta, Bangladesh. Egyptian Journal of Aquatic Research, 41(4), 307– 313. doi:10.1016/j.ejar.2015.11.001.
- Tripathy SK (2020) Significance of Traditional and Advanced Morphometry to Fishery Science. Journal of Human, Earth, and Future Vol. 1, No. 3, September, 2020 http://dx.doi.org/10. 28991/HEF-2020-01-03-05.
- Strauss RE, Bond CE (1990) Taxonomic methods: morphology. In: Schreck CB, Moyle PB (eds) Methods for fish biology. American Fisheries Society, Maryland, pp 109–140
- Uiblein F (1995) Morphological variability between populations of Neobythites stefanovi (Pisces:Ophidiidae) from the deep Red Sea and the Gulf of Aden. Marine Ecology Progress Series, 124, 23–29. doi:10.3354/meps124023.
- Sarder MRI, Simonsen V (2006). Final report on the study and workshop on causes for and consequences of reduced growth in Indian major carps (p. 62).
- Hurlbut T, Clay D (1998). Morphometric and meristic differences between shallow- and deep-water populations of white hake (Urophycis tenuis) in the southern Gulf of St. Lawrence. Canadian Journal of Fisheries and Aquatic Sciences, 55(10), 2274–2282. doi:10.1139/f98-110.
- Turan C (2004).Stock identification of Mediterranean horse mackerel (Trachurus mediterraneus) using morphometric and meristic characters. ICES Journal of Marine Science, 61(5), 774–781. doi:10.1016/j.icesjms.2004.05.001.
- Teletchea F (2009)Molecular identification methods of fish species: reassessment and possible applications. Rev Fish Biol Fisheries (2009) 19:265–293 DOI 10.1007/s11160-009-9107-4.
- Allendorf FW, Phelps SR (1988) Loss of genetic variation in hatchery stock of cutthroat trout. Trans. Am. Fish. Soc. 109: 537-543.
- Wimberger PH (1992) Plasticity of fish body shape - the effects of diet, development, family and age in two species of Geophagus (Pisces: Cichlidae). Biol. J. Linn. Soc. 45: 197-218.
- Swain DP, Ridell BE, Murray CB (1991) Morphological differences between hatchery and wild populations of coho salmon (Oncorhynchus kisutch): environmental versus genetic origin. Can. J. Fish. Aquat. Sci. 48: 1783-1791.
- Hossain MAR, Nahiduzzaman M, Saha D, Habiba Khanam MU, Alam MS (2010). Landmark-based morphometric and meristic variations of the endangered carp, kalibaus Labeo calbasu, from stocks of two isolated rivers, the Jamuna and Halda, and a hatchery. Zoological Studies, 49(4), 556–563.
- Juliao (2020) https://agencia.fapesp.br/environmental-factors-influence-fish-morphology-and-behavior/32635/.
- Radojkovic N, Marinovic Z, Miloskovic A , Radenkovic M , Duretanovis S, Lujic J, Simic V (2018) Effects of Stream Damming on Morphological Variability of Fish: Case Study on Large Spot Barbell Barbus balcanicus.
- Eklöv P, Svanbäck R (2006). Predation risk influences adaptive morphological variation in fish populations. In American Naturalist (Vol. 167, Issue 3). American Nationals. doi:10.1086/499544.
- Keskin E, Atar HH. DNA barcoding commercially important fish species of T urkey. Molecular ecology resources. 2013 Sep;13(5):788-97.
- Cohen NJ, Deeds JR, Wong ES, Hanner RH, Yancy HF, White KD, Thompson TM, Wahl M, Pham TD, Guichard FM, Huh I, Austin C, Dizikes G, Gerber SI (2009) Public health response to pufer fsh (tetrodotoxin) poisoning from mislabeled product. J Food Prot 72:810–817
- Bashir A, Bisht SB, Mir IJ, Patiyal SR , Kumar R (2016) Morphometric variation and molecular characterization of snow trout species from Kashmir valley, India.Mitochondrial DNA part A DNA Mapping, Sequencing, and Analysis Volume 27, 2016 – Issue 6.
- Straüss RE, Bond CE. In: Methods for Fish Biology, 1990. Amer Fish Soc. Schreck CB, Moyle PB, editors. Maryland, U.S.A: Bethesda; 1990. Taxonomic methods: morphology; pp. 125–130.
- Ko HL, Wang TY, Chiu TS, Lee MA, Leu MY, Chang KZ, ChenWY, Shao KT (2013) Evaluating the Accuracy of Morphological Identification of Larval Fishes by Applying DNA Barcoding, PLoS ONE 8(1): e53451. https://doi.org/10.1371/journal.pone.0053451.
- Zhang JB, Hanner R. DNA barcoding is a useful tool for the identification of marine fishes from Japan. Biochemical Systematics and Ecology. 2011 Feb 1;39(1):31-42.
- Uiblein F. Morphological variability between populations of Neobythites stefanovi (Pisces: Ophidiidae) from the deep Red Sea and the Gulf of Aden, Marine ecology progress series.vol 124:23-29,1995.
- Tillett BJ, Field IC, Bradshaw CJA, Johnson G, Buckworth RC, Meekan M G, Ovenden JR(2012). Accuracy of species identification by fisheries observers in a north Australian shark fishery. Fisheries Research, 127-128, 109-115. https://doi.org/10.1016/j.fishres.2012.04.007
- CohenJN, DeedsRJ, WongSE, HannerHR, YancyFH, WhiteDK, ThompsonMT, WahlI, PhamDT, GuichardMF, HuhI, Chandra S, Barat A, Singh M, Singh K B, Matura R (2012) DNA Bar-Coding of Indian Coldwater Fishes of Genus Schizothorax (Family: Cyprinidae) from Western HimalayaWorld Journal of Fish and Marine Sciences 4 (4): 430-435, 2012
- Austin C, Dizikes G, Gerber IS (2009) Public Health Response to Puffer Fish (Tetrodotoxin) Poisoning from Mislabeled Product J Food Prot (2009) 72 (4): 810–817.
- Ghouri MZ, Ismail M, Javed MA, Khan SH, Munawar N, Umar AB, Nisa M, Aftab SO, Amin S, Khan Z and Ahmad A (2020) Identification of Edible Fish Species of Pakistan Through DNA Barcoding. Front. Mar. Sci. 7:554183. doi: 10.3389/fmars.2020.554183
- Cawthorn MD, Steinman AH, Witthuhn CR (2012) DNA barcoding reveals a high incidence of fish species misrepresentation and substitution on the South African market.Food research international Volume 46, Issue 1Pages 1-436.
- Vartak RV, Rajendran N, Lakra SW (2014) DNA barcoding: Importance in fisheries research and food safety, BioTechnology An Indian Journal volume 9.
- Callejas C, Ochando MD (2001) Molecular identification (RAPD) of the eight species of the genus Barbus (Cyprinidae) in the Iberian Peninsula. J Fish Biol 59:1589–1599. doi:10.1111/ j.1095-8649.2001.tb00223.x.
- Gewin V (2002) All living things, online. Nature 418: 362-363.
- Wilson EO (2003) What is Nature Worth For? Part 1: Span ZLIV-4: 54-57 Part 2: XLIV, 23-29.
- Simonetti JA (1997) Biodiversity and a taxonomy of Chilean taxonomists. Biodiversity and Conservation 6: 633-637.
- Tahseen Q (2014) Taxonomy-The Crucial yet Misunderstood and Disregarded Tool for Studying Biodiversity. J Biodivers Endanger Species 2: 128. doi:10.4172/2332-2543.1000128
- Wheeler QD, Raven PH , Wilson EO (2004) Taxonomy: Impediment or expedient? Science, 303 (5656), 285.
- De Carvalho MR, Bockmann FA, Amorim DS, de Vivo M, et al. (2005). Revisiting the taxonomic impediment. Science, 307, 353.
- Talwar PK, Jhingren AG (1991) Inland Fishes of India and adjacent Countries. Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi. Vol. II:115-116.
- Jayaram, K C (2010). Freshwater fishes of the Indian region. Narendra Pub. House.
- Sarma K J, Mankodi P C (2017). Deciphering identification of inland fishes of Gujarat using dna barcoding. Turkish Journal of Fisheries and Aquatic Sciences. 17. 1059-1061. 10.4194/1303-2712-v17_5_22.
- Hebert PD, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proc Biol Sci. 2003 Feb 7;270(1512):313-21. doi: 10.1098/rspb.2002.2218. PMID: 12614582; PMCID: PMC1691236.
- Brasier MJ, Wiklund H, NealL, Jeffreys R, Linse K, Ruhl H, Glover AG (2016)DNA barcoding uncovers cryptic diversity in50% of deep-sea Antarctic polychaetes.R.Soc.opensci.3: 160432.
- Korshunova T, Picton B, Furfaro G et al. (2019)Multilevel fine-scale diversity challenges the ‘cryptic species’ concept. Sci Rep 9, 6732 (2019). https://doi.org/10.1038/s41598-019-42297-5
- The Global Taxonomy Initiative .2020: A Step-by-Step Guide for DNA Barcoding
- Kochzius M, Seidel C, Antoniou A, Botla SK, Campo D, Cariani A, et al. (2010) Identifying Fishes through DNA Barcodes and Microarrays. PLoS ONE 5(9): e12620.
- Fischer J (2013) Fish identification tools for biodiversity and fisheries assessments: review and guidance for decision-makers. FAO Fisheries and Aquaculture Technical Paper No. 585. Rome, FAO. 2013. 107 pp.
- Gregory TR. DNA barcoding does not compete with taxonomy. Nature. 2005 Apr 28;434(7037):1067. doi: 10.1038/4341067b. PMID: 15858548.
- Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN. DNA barcoding Australia's fish species. Philosophical transactions of the Royal Society of London. Series B. 2005; 360(1462):1847-1857. https://doi.org/10.1098/rstb.2005.1716.
- Moura T, Silva MC, Figueiredo I, Neves A, Munoz PD, Coelho MM and Gordo LS (2008) Molecular barcoding of north-east Atlantic deep-water sharks: species identification and application to fisheries management and conservation Marine and Freshwater Research 59(3) 214-223
- Hubert N, Hanner R, Holm E, Mandrak NE, Taylor E, et al. (2008) Identifying Canadian Freshwater Fishes through DNA Barcodes. PLOS ONE 3(6): e2490. https://doi.org/10.1371/journal.pone.0002490
- Holmes, Bronwyn, Steinke, Dirk, Ward, Robert. (2009) Identification of shark and ray fins using DNA barcoding. Fisheries Research. 95. 280-288. 10.1016/j.fishres.2008.09.036.
- Steinke D, Zemlak TS, Boutillier JA, Hebert PND (2009)DNA barcoding of pacific canadas fishes, Marine Biology. November 2009 156:2641–2647
- Ahmed MS, Dina SR, Nahar L, Islam NN, Reza HA (2018) Molecular characterization of Channa species from Bangladesh based on Cytochrome C Oxidase Subunit I (COI) gene FishTaxa (2018) 3(4): 87-93.
- Lakra SW, Verma MS, Goswami M, Lal KK, Mohindra V, Punia P, Gopalakrishnan AK, Singh KV, Ward RD, Hebert P (2011) DNA barcoding Indian marine fishes. Molecular Ecology Resources (2011) 11, 60–71
- Chandra S ,Barat A ,Singh M, Singh KB, Matura R (2012) DNA Bar-Coding of Indian Coldwater Fishes of Genus Schizothorax (Family: Cyprinidae) from Western HimalayaWorld Journal of Fish and Marine Sciences 4 (4): 430-435, 2012
- Barman D, Roy S, Kumar V, Mandal SC, Kumar V (2012) DNA barcoding applications in aquaculture, Global Aquaculture Advocate Saturday, 3 March 2012.
- Khedkar GD, Jamdade R, Naik S, David L, Haymer D (2014). DNA barcodes for the FIshes of the Narmada, one of India’s longest rivers. PLoS ONE., 9(7)
- Thapliyal M, Pokhriyal H, K Sati B, Nagpure N, Singh M, and Thapliyal A (2015) Molecular characterization of coldwater fishes of district Uttarkashi, Uttarakhand using DNA Barcoding Environment Conservation Journal 16(3) 109-116.
- Kumar S, Singh D.Genetic and morphometric comparison of two isolated populations of Barilius bendelisis (Cypriniformes: Cyprinidae) from the Indian Himalayas. Revista de Biología Tropical vol. 67, no. 3, 2019, pp. 466-477
- Kundu S, Chandra K, Tyagi K, Pakrashi A, Kumar V (2019) DNA barcoding of freshwater fishes from Brahmaputra River in Eastern Himalaya biodiversity hotspot . Mitochondrial DNA part B 2019, vol. 4, no. 2, 2411-2419.
- Alam A, Chadha KN, Kumar PA, Chakraborty KS, Joshi DK, Sawant BP, Das SCS, Kumar J, KumarT (2019) DNA Barcoding and Biometric Investigation on the Invasive Oreochromis niloticus (Linnaeus, 1758) from the River Yamuna of Uttar Pradesh.Indian Journal of Animal Research.2020.(54):856-863.
- Kashmiri Pandit network: Geography of Jammu and Kashmir State, http://ikashmir.net/geography/chapter1.1.html
- Nasim and keng (2012) Directory of lakes and waterbodies of j&k state using remote sensing & GIS technology. department of environment and remote sensing sda colony bemina srinagar / paryawaran bhawan, forest complex, jammu.
- Heckel JJ (1838) Fische aus Caschmir. Karl Freiherrn von Hugel. Wien. Gedruckt Bei Den P. P. Mechitaristen. 112pp+13pl
- Day F (1876) On the fishes of Yarkand. Proc Zoology Soc London:781–807
- Silas E G (1960) Fishes from Kashmir Valley. J Bombay Nat Hist Soc 57(1):145–182
- Das SM, Subla BA (1964) The Ichthyofauna of Kashmir, Part II. The speciation of Kashmir fishes. Ichthyologica 3:57–62.
- Das SM, Nath S (1965) The ichthyofauna of Poonch Valley (J&K). Kashmir Sci 2(1–2):149–155
- Yousuf AR (1996) Fishery resource of Kashmir. In: Khan AH, Pandit AK (eds) Ecology, Environment and Energy. Kashmir University, Srinagar, pp 75–120
- Kullander SO, Fang F, Delling B, Ahlander E (1999) The fishes of the Kashmir Valley. pp. 99-162. In: Lennart Nyman (Ed.), River Jhelum, Kashmir Valley, impact on the aquatic environment SWEDMAR, Göteborg.
- Enderlin O, Yousuf AR (1999) The environmental impacts f the Uri hydropower project on the fish community in the river Jhelum. In: Nyman L (ed) River Jhelum, Kashmir valley: impacts on the aquatic environment. SWEDMAR, Göteborg, pp 169–186
- Balkhi MH (2007) Fish diversity of Jammu & Kashmir and its conservation. In: Patloo (ed) Kashmir speaks. G. M. Publishers, Kashmir, pp 104–118
- Khan I, Ali M (2013)Current Status of the Fish Fauna of River Jhelum, Kashmir, J&K, Open Access Scientific Reports 2: 694
- Jan R , Gupta R , Najar MA and Zuber SM (2015) Abiotic Status of River Jhelum with Special Reference to its Ichthyofaunal Diversity Research Journal of Animal, Veterinary and Fishery Sciences. ISSN 2320 – 6535
- Ahmed I, Ahmad Z, Ahmad I (2017) Current status of fish fauna of river Jhelum and dal lake of kashmir valley Bulletin of Pure and Applied Sciences .Vol 36 A (Zoology), No.2, P.85-92.
- Bahir M, Chauhan R , Mir FM, Ashraf M, Amin N(2016) Effect of pollution on fish diversity in Anchar lake Kashmir,International journal of fisheries and aquatic resources 2017; 5(1): 105-107
- Brraich SO, Malik AI (2016) Fish fauna of Wular Wetland (Ramsar site in Kashmir Himalayas, India) Iran. J. Ichthyol. (September 2016), 3(3): 218–221.
- Rumysa K, Sharique AA, Bilal A, Tariq Z and Farooq M (2016) On the fish diversity, conservational management and rehabilitation aspects of Wular Lake, Kashmir India. Ecological Communication.Biosci. Biotech. Res. Comm. 9(4): 872-877.
- Qadri S, Bhat FA, Balkhi MH, Shah TH, Hussain N, Bhat AB, Altaf M, and Aalia S (2018) Fish biodiversity, catch composition, distribution pattern of fishes in Wular Lake and their conservational measures for sustainable fish production. Journal of Pharmacognosy and Phytochemistry 2018; 7(6): 1883-1887.
- Mushtaq AS, Balkhi MH, Abubakr A, Kumar A, Shah HT, Ahmed B, Shah F, Talia S, QadriS, Farooq I (2020) Current status of fish fauna, catch composition of Hokersar Wetland, Kashmir, International Journal of Fauna and Biological Studies 2020; 7(1): 15-18
- Hamid J, Singh RK (2019) Diversity of Fishes in River Viashaw: the left tributary of river Jhelum of Jammu and Kashmir, India, JETIR may 2019, volume 6, issue 5.
- Rashid G, Singh R.(2020)Present status of fish diversity and their conservational measures for sustainable fishproduction in the vaishav stream of kashmir himalaya, india, Journal of critical reviews,vol 7, issue 16, 2020
- Akhtar T, Ali G (2016) DNA barcoding of Schizothorax species from the Neelum and Jhelum Rivers of Azad Jammu and Kashmir Mitochondrial DNA Part B · December 2016.
- Bashir A, Bisht BS, Mir JI, Kumar R, Patiyal RS. Morphological, molecular characterization and taxonomic status of Triplophysa marmorata and Triplophysa kashmirensis (Cypriniformes: Nemacheilidae) from Kashmir valley, India. Rev Biol Trop.2016 Jun;64(2):473-82. doi: 10.15517/rbt.v64i2.19591. PMID: 29451748.
- Das SM and Nath S (1966) The icthyofauna of Jammu province (J&K), Kashmir Sci.,2 (1-2) : 65-78.
- Das SM and Nath S (1971) A revision of the fishes from Jammu province, Kashmir Sci., 7: 1-12.
- Malhotra YR, Jyoti MK, Dutta SPS (1975) An aid to the identification of fishes found in Jammu division of J&K State. Jammu Univer Rev 5:50–66
- Joshi CB, Sehgal K L, Sunder S (1981) Observations on the fishery resources of the hill streams of Jammu Province with special reference to mahseer and other commercially important species. Indian journal of fisheries
- Tilak 1971. The fishes of river tawi and its r-tributaries (jammu & kashmir state) with notes on ecology.Zoological Survey of India, Calcutta 183-217.
- Dutta SPS and Malhotra YR (1984) An upto date checklist and a key to identification of fishes of Jammu. Jammu Univ. Review., 2 : 65-92.
- Jyoti MK, Sharma K, Gupta K, Baba DI (2006). Aquatic biodiversity: a review of freshwater flora and fauna of J&K State. In: Jyoti MK, Sharma KK, Gupta K (eds) Proceeding National Symposium: status of coldwater fishes with reference to fragile Himalayan aquatic ecosystems. Classic Printers, Bari Brahmna, pp 258–292
- Baba DI, Sharma KK, & Jasrotia S (2014) Ichthyofaunal Diversity of River Chenab: A Case Study. International Journal Of Lakes and Rivers, 55-62.
- Bhutyal R, Langer S (2015) Current status of fish fauna of river Chenab, in kishtwar district, j&k, Asian academic research journal of multidisciplinary,Issue 5.
- Gandotra R, Zaman U R, Vivek (2017). Longitudinal Patterns of Population Structure for Fishes Inhabiting River Tawi in Jammu region (J&K),Biosci.,Biotech. Res. Asia,Vol.14(3), 1121-1127. http://dx.doi.org/10.13005/bbra/2550
- Sharma A, Dutt SPS (2012) Present and past status of fish fauna of river Basantar, an important tributary of the river Ravi, in Samba district, Jammu (J&K). Journal of Applied and Natural Science 4 (1): 123-126
- Vohra A, Jyoti MK, Gupta K (2013)Some ornamental fishes of Jammu region International journal of innovative research and development. Vol 2 Issue 8: 379-390.
- Arif M, Gandotra R, Choudhary N, Sharma D, Lone A, Dogra A (2019) Study on Distribution and Abundance of Indigenous Ornamental Fishes from Water Bodies of Jammu (J&K), Biosciences Biotechnology Research Asia. Vol. 16(2), p. 483-489
- Dutta SPS (2015) Survey and systematic analysis of Fish fauna of erstwhile Udhampur District, Jammu region, with new records of Barilius radiolatus Gunther, Botia lohachata Chaudhari, Botia dario (Ham. Buch.) and Glyptothorax punjabensis Mirza and Kashmiri,Environment Conservation Journal 16(3) 39-47, 2015.
- Rathore V, Dutta SPS (2015) Fish fauna of river Ujh, an important tributary of the river Ravi, District Kathua, Jammu, Environment Conservation Journal 16 (1&2)81-86.
- Khajuria B, Langer S, Khan S (2015) Icthyodiversity and Species Richness of Chadwal Stream, Kathua District (Jammu & Kashmir), International journal of scientific research. Volume - 4 | Issue : 10,63-65.
- Gandotra R, Sharma P (2015) Study of Ichthyofaunal Diversity in a stream in Sunderbani, District Rajouri, Jammu (J&K),International Journal of Multidisciplinary Research and Development Volume: 2, Issue: 9, 401-404.
- Kour H , Sharma KK, Sharma A (2015) Icthyofaunistic survey of some lotic water bodies of R.S Pura, Tehsil, Jammu, Journal of International Academic research for Multidisciplinary, 3: 2320-5083.
- Dutta SPS (2016) Fish fauna of Wajoo nullah, an important tributary of the river Ravi in Kathua District, Jammu Region, Jammu and Kashmir State, India. Journal of Applied and Natural Science 8 (2): 1087 - 1089
- Hussain S, Dutta SPS (2016)Fish fauna of tehsil mendhar, district- poonch, jammu region, j&k state, with a new record of botia birdi, ompok pabda and glyptothorax punjabiensis from poonch district, Proc.Zool.Soc.India. 15 (1) : 1 – 11
- Khajuria B, Langer S, Kumar S, Choudhary A(2016).Fish diversity and habitat relationship of selum nullah and Aik nullah with reference to physico chemical parameters at Bari Brahmana,Jammu district,JandK.Journal of advanced scientific research,2016,7(1):53-59
- Nisa RU, Gupta K, Wani SM, Allie KA, kouser N (2020) Study on diversity and ecology of ichthyofauna of Rajouri district, Jammu and Kashmir, India. Rec. zool. Surv. India: Vol. 120(4):363–372
- Dutta SPS (2021) Fish Fauna of the River Ravi and Its Some Tributaries with a New Record of Ailia puncata and Clupisoma naziri for Punjab State and Union Territory of Jammu and Kashmir, India. Aquaculture and Fisheries Studies.vol3,issue1:1-5.
- Gupta SC and Dutta SPS (2021) Fish Fauna of River Sewa, an Important Himalayan Tributary of the River Ravi, in Kathua District of Union Territory of Jammu & Kashmir, India, biomedical journal of scientific and technical research. Volume 36- Issue 3:28474-28478.
- Arif M and Gandotra R (2017) DNA barcoding of ornamental fishes of Jammu:7th international barcode of life conference 2017 nov.
- Kadye WT, Moyo NAG, Magadza CHD,Kativu S (2008)Stream fish assemblages in relation to environmental factors on a Montane Plateau(Nyika Plateau,Malawi).Environmental biology of fishes,83,417-423.
- Makori AJ, Abuom PO, Kapiyo R,et al.(2017) Effects of water physico-chemical parameters on tilapia (Oreochromis niloticus) growth in earthen ponds in Teso North Sub-County, Busia County. Fish Aquatic Sci 20, 30. https://doi.org/10.1186/s41240-017-0075-7
- Krishan R (2016)Impact of climate change on fish fauna of jammu and Kashmir,International journal of innovative research in science and engineering.vol no 2,Issue 03,March .
- Bhat FA, Yousuf AR, Balkhi MH (2020). Diversity of Fishes in Jammu and Kashmir State. In: Dar, G., Khuroo, A. (eds) Biodiversity of the Himalaya: Jammu and Kashmir State . Topics in Biodiversity and Conservation, vol 18. Springer, Singapore. https://doi.org/10.1007/978-981-32-9174-4_33
- Andotra P (2014) Impact of pollution on water quality and fishes of river Tawi. Ph.D.Thesis, University of Jammu, Jammu 2014.
- Bhatt JP, Pandit KM (2015) Endangered Golden mahseer Tor putitora Hamilton: a review of natural history,Rev Fish Biol Fisheries 26:25–38
- Sharma KA, Thakur NS (2017) Assessing the impact of small hydropower projects in Jammu and Kashmir: A study from north-western Himalayan region of India






