Determination of Heavy Metal (Cu, Pb And Zn) Concentrations in Muscle Tissue of Hypophthalmichthys Molitrix, Cyprinus Carpio and Ctenopharyngodon Idella Caught From Zarivar Wetland, Western Iran
Seyed Milad Jafari1 and Soheil Sobhanardakani2 *
1
Young Researchers and Elite Club,
Hamedan Branch,
Islamic Azad University,
Hamedan,
Iran
2
Department of the Environment,
College of Basic Sciences Hamedan Branch,
Islamic Azad University,
Hamedan,
Iran
DOI: http://dx.doi.org/10.12944/CWE.9.3.44
Copy the following to cite this article:
Jafari S. M, Sobhanardakani S. Determination of Heavy Metal (Cu, Pb And Zn) Concentrations in Muscle Tissue of Hypophthalmichthys Molitrix, Cyprinus Carpio and Ctenopharyngodon Idella Caught From Zarivar Wetland, Western Iran. Curr World Environ 2014;9 (3) DOI:http://dx.doi.org/10.12944/CWE.9.3.44
Copy the following to cite this URL:
Jafari S. M, Sobhanardakani S. Determination of Heavy Metal (Cu, Pb And Zn) Concentrations in Muscle Tissue of Hypophthalmichthys Molitrix, Cyprinus Carpio and Ctenopharyngodon Idella Caught From Zarivar Wetland, Western Iran. Curr World Environ 2014;9(3). Available from: http://cwejournal.org?p=538/
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2014-06-22 |
|---|---|
| Accepted: | 2014-07-29 |
The presence of metals in aquatic ecosystems originates from the natural interactions between the water, sediments and atmosphere (Kalay and Canil, 2000; Sankar et al. 2006). Heavy metals may enter an aquatic ecosystem from different natural and anthropogenic sources, including industrial or domestic sewage, storm runoff, leaching from landfills, shipping and harbor activities and atmospheric deposits (Nair et al. 2006). The contamination of heavy metals in the various parts of organisms are determined primarily indicative of the level of the pollution in the environment (Canbek et al. 2007). Aquatic organisms are widely used to monitor environmental health due to anthropogenic impacts (Hellawell, 1986; Evans et al. 1993; Rashed, 2001; Rajeshkumar and Munuswamy, 2011).
Of many aquatic organisms, fish is a valuable biomonitor of environmental pollution (Padmini and Usha, 2008). Chronic contamination by heavy metals and organic pollutants in the marine environment is a severe problem particularly in estuaries. This has prompted numerous investigations on the effects of these pollutants on the biological functions of aquatic organisms and in particular defense mechanisms in fish (Wood, 1991). Its complexity and constant contact with the external environment make the gill the first target to waterborne pollutants (Mallatt, 1985; Perry and Laurent, 1993; Fracacio et al. 2003). The effect of different contaminants on gill and liver causing biochemical and morphological changes have been analyzed in several studies (Monterio et al. 2005; Garcia-Santos et al. 2006; Romao et al. 2006; Fernandes et al. 2007). Several studies were done to measure and determine the effects of heavy metals and trace elements on ecosystem and human. The studies showed that decreased content of antioxidative elements, such as Zn, Se and Mn and increased content of some elements including Cu, Co and As, which probably elevate the oxidative stress, can cause some inflammatory diseases and cardiac functional disorders (Barandier et al. 1999; De-lorgeril et al. 2001; Salehifar et al. 2008; Shokrzadeh et al. 2009; Topuzoglu et al. 2003). On the other hand, Pb toxicity can lead to growth retardation, neuronal defects and anemia in children. Also, hepatotoxicity, nephrotoxicity and neurotoxicity can be occurred following Pb chronic toxicity (Tabari et al. 2010).
Cu under ionic forms Cu2+, Cu2OH+ and CuOH+ is toxic to fish (Ashraf et al. 2006). Zn is present in many enzymes involved in important physiological functions like protein synthesis and constitutes about 33 ppm of adult body weight (Ashraf et al. 2006). Pb poisoning is generally ranked as the most common environmental health hazard (Goyer, 1994). Pb absorption may constitute a serious risk to public health. Pb may induce reduced cognitive development and intellectual performance in children and increased blood pressure and cardiovascular diseases in adults. Over the past decade the levels in food have decreased significantly owing to the awareness of lead as a health problem and source related efforts to reduce the emission of Pb (Suppin et al. 2005).
Zarivar Lake (ZL) is fresh water body with an area of about 750 ha and average water depth of 4-5 meters in far west of Iran located in 35°-30’ to 35°-35’ North and 46°-06’ to 46°-09’ East in the North of Kurdistan province, Iran (Figure 1). Zarivar Lake is a typical ecosystem of great importance in regard to biodiversity and to aesthetic value. The fish species found most commonly in the lake are Cyprinus carpio, Ctenopharyngodon idella, Hypophthalmichthys molitrix, Capoeta damascina, Pseudorasbora parva, Chalcalburnus sp, Carassius auratus, Gambusia affinis and Mastacembelus mastacembelus. Previous research showed that the pollutants transferred to ZL and regarding the intensity of pollution production, the non point source pollution related to agricultural activities was first rank among other pollutant as community wastewater, solid waste, grassland pollution and forest. These pollutions are transferred directly to wetland and threaten the biological systems of ZL (Ghaderi and Ghafouri, 2006).
The aim of this study was to provide baseline information on heavy metal (Cu, Pb and Zn) contamination in muscle tissue of three fish species from ZL, to determine whether these metals are within permissible limits for human consumption.
 |
Figure 1: Location of Zarivar Lake in Marivan city, Western Iran Click here to View Figure |
Materials and Methods
Chemical and reagents
All chemical reagents were of analytical reagent grade, purchased from Merck (Germany). All solutions were prepared with doubly distilled water. Stock standard solution of Cu(II), Pb(II), and Zn(II) (1,000 mg L-1) were prepared by dissolving the appropriate amount of metal salts in doubly distilled water and diluting to 1,000 ml in the volumetric flask. As supporting electrolyte, 0.1 M acetate–acetic acid buffer (pH= 4.5) was used.
Apparatus
All voltammetric measurements were carried out using a polarographic processor, model 746 VA (Metrohm), in combination with a polarographic stand model 747 VA (Metrohm). The electrode stand consists of a hanging mercury drop electrode (HMDE) as working electrode, a double junction Ag/AgCl (3 M KCl, saturated AgCl, and 3 M KCl in the bridge) as reference electrode, and platinum wire, with considerably larger surface area than that of HMDE, as auxiliary electrode. All potentials quoted are relative to the Ag/AgCl reference electrode. Stirring was carried out by a large Teflon road with 2,000 rpm speed. A 780 pH Meter (Metrohm), equipped with a combined Ag/AgCl glass electrode was used for pH measurements. Eppendorf reference variable micropipettes were used to pipette microliter volumes of solutions. All glasswares were soaked overnight in 10% (v/v) nitric acid, followed by washing with 10% (v/v) hydrochloric acid, and rinsed with doubly distilled water and dried before using.
Sample preparation
Although voltammetric techniques are inherently precise and accurate, the results obtained using these techniques may be invalidated due to contamination caused by poor sample handling and preparation. Therefore, stringent conditions should be routinely used for trace analysis. In this study fish samples were cleaned with distilled water and then dissected. 2 g of muscle tissue of each fish sample was removed and weighed for the analysis. For estimation of heavy metal content, 2 g of each tissue was taken in a 100-ml Borosil beaker. To this, 2 ml of HNO3 and 1 ml of HClO4 were added and kept for digestion on a hot plate at 100°C till complete digestion was achieved. It was ensured that the residue obtained after digestion was free from organic matter which acts as impurities in metal analysis (Sobhanardakani et al. 2011a).
Sample analysis
To analysis of Cu(II), Pb(II) and Zn(II) concentrations in the muscle of fish species (Hypophthalmichthys molitrix, Cyprinus carpio and Ctenopharyngodon idella), 5 ml of each sample solution and 1 ml acetate–acetic acid buffer solution were transferred into the electrochemical cell and diluted to 10 ml by doubly distilled water. The solution was deaerated by passing pure nitrogen for 5 min. The deposition potential was controlled at (-0.25, -0.75 and -1.0 for Cu, Pb and Zn respectively) and applied to a fresh mercury drop while the solution was stirred. After the deposition step and further 10 s (equilibrium time), the voltammograms were recorded. Different concentrations of the standard metal ions were added to the cell. The solution was stirred and purged with nitrogen for 1 min after each spike. Finally, the concentrations of Cu(II), Pb(II) and Zn(II,) were calculated in the sample solutions by using the standard addition method (Sobhanardakani et al. 2011a; Sobhanardakani et al. 2011b).
Statistical analyses
Statistical analysis was performed using SPSS 15.0 version (SPSS Inc., Chicago, IL, USA) statistical package. Data were grouped according to species. One-way analysis of variance was used to test for differences in tissue metal concentrations. Data were log-transformed to improve normality before analysis to meet the underlying assumptions of the analysis of variance; the values given are therefore geometric means. The differences between the metal concentrations in different species were analyzed using the t-test. Possibilities less than 0.05 were considered statistically significant (p < 0.05).
Results and discussions
Because of high sensitivity of anodic stripping voltammetry, this method is applied to the determination of Cu, Pb and Zn in the muscle tissue of fish species (Hypophthalmichthys molitrix, Cyprinus carpio and Ctenopharyngodon idella).
The concentrations of Cu, Pb and Zn in fish species are presented in Table 1 along with the statistical parameters. Statistical analysis of the data for all fish species showed significant differences among all of the samples. Figure 2 shows the comparative levels of Cu, Pb and Zn in the various species of fish. It can be seen that the average concentrations Cu in grass carp is more than 2 times higher than common carp. Similarly the Pb for grass carp is much higher than common carp as well. On the other hand, the average Zn content in grass carp was much lower than in common carp. Statistical grouping of the concentrations of each element in the different species of fish by ANOVA and the Tukey test are shown in table 1. The results indicated that there were significant differences within and between all of the evaluated brands (p<0.05).
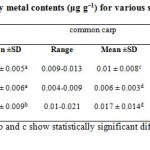 |
Table 1: Heavy metal contents (μg g−1) for various species of fish Click here to View table |
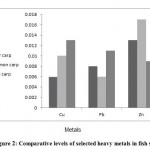 |
Figure 2: Comparative levels of selected heavy metals in fish species Click here to View Figure |
The levels of heavy metals in fish depend upon many factors like the duration of exposure of fish to contaminants in the water, the feeding habits of each fish species, the, concentrations of contaminants in the water column, water chemistry, any contamination of fish during handling and processing, and fish sex, weight, season (Kagi and Schaffer, 1998; Boadi et al. 2011).
It is known that seafood is a good source of dietary Cu, which is an essential element for humans but where a very high intake (>120 μg g−1) can again cause adverse health problems, such as liver and kidney damage (WHO, 1996; Aucoin et al. 1999; Ikem and Egeibor, 2005). Whereas the maximum permitted Cu level established by the FAO (1983) and WHO (1996) is 30 μg g−1 and 20 μg g−1 by MAFF (1993), the, literature values ranged from 0.23 to 9.49 μg g−1 for muscles of fish from the Marmara Sea (Keskin et al. 2007), 0.7-27 μg g−1 for muscles of fish from Lake Budi (Tapia et al. 2006), 0.74-2.24 μg g−1 for muscles of fish from Iskenderun Bay and 0.32-6.48 μg g−1 for muscles of fish from the Marmara, Aegean and Mediterranean Seas (Turkmen et al. 2006; Turkmen et al. 2008), 0.15-5.06 μg g−1 for muscles and whole fish from Turkish Seas (Tepe et al. 2007), 0.001-6.29, 0.001-57.3, 0.001-15.9 and 0.08-32.9 μg g−1 for muscle tissues of anchovy, red mullet, mackerel and picarel respectively from the Croatian waters of the Adriatic Sea (Bilandzˇic´ et al. 2011), 0.157 and 1.206 μg g−1 for muscle tissue of milk fish from less polluted and polluted sites of Kaattuppalli Island, India respectively (Rajeshkumar and Munuswamy, 2011), Sobhanardakani et al. reported that Cu in the muscle tissue of five fish species (Otolithes ruber, Pampus argenteus, Parastromateus niger, Scomberomorus commerson, Onchorynchus mykiss) ranged from 0.007-0.23 μg g−1 (Sobhanardakani et al. 2011b).
The maximum Pb level permitted reported by the WHO (1996) is 2.0 μg g−1, 0.5 μg g−1 by the FAO (1983) and 2.0 μg g−1 by the MAFF (1995). Eboh et al. (2006) reported that Pb in the muscle, gills and liver tissue of five common commercially available fish species in Nigeria (catfish, tilapia, ilisha, bonga and mudskipper) were found in the range of 0.001-0.002 μg g−1 but did not find any heavy metal residues in salmon and mackerel species. On the other hand, the mean concentration of Pb (4.27-6.12 μg g−1) reported by Canli and Atli (2005) in muscle tissues of six different fish species (Sparus auratus, Atherina hepsetus, Mugil cephalus, Trigla cuculus, Sardina pilchardus and Scomberesox saurus), Rajeshkumar and Munuswamy (2011) reported that Pb in the muscle tissue of milk fish from less polluted and polluted sites of Kaattuppalli Island, India were found in the range of 0.035 and 0.058 μg g−1 respectively, Bilandzˇic´ et al. (2011) reported that Pb in the muscle tissues of anchovy, red mullet, mackerel and picarel from the Croatian waters of the Adriatic Sea in the range of 0.001-0.34, 0.001-0.27, 0.002-0.24 and 0.001-0.46 μg g−1, respectively. Tabari et al. (2010) reported that the concentration of Pb in the muscle tissues of three species (Cyprinus carpio, Mugila auratus and Rutilus frisikutum) at 12 fishing site form Southern Caspian Sea, Iran in the range of 53.7 to 168.9 μg g−1, Sobhanardakani et al. (2011a) reported that Pb in the muscle tissue of five fish species (Otolithes ruber, Pampus argenteus, Parastromateus niger, Scomberomorus commerson, Onchorynchus mykiss) ranged from 0.007-0.09 μg g−1.
Turkmen et al. (2009) determined the metal levels in the muscle of 12 fish species from the Aegean and Mediterranean Seas and reported that the level of Zn in muscle of fish was 3.51-53.5 μg g−1. Sivaperumal et al. (2007) reported that Zn in the muscle tissue of 23 fish species were obtained from internal markets of India ranged from 0.66-39.2 μg g−1. Yilmaz et al. (2007) analyzed Zn in the muscle of two fish species (Leuciscus cephalus and Lepomis gibbosus) caught from Saricay, South-West Anatolia in the range of 6.35-28.55 μg g−1. Yilmaz (2009) analyzed Zn in the muscle of 127 fish samples of three fish species (Anguilla anguilla, Mugil cephalus, Oreochromis niloticus) caught from KöyceÄŸiz Lake- Mugla in Turkey. In their study the lowest metal contents were found in the edible parts (muscle) of all species. However, Zn for O. niloticus; Zn for A. anguilla; and Zn for M. cephalus were higher than those established by Turkish Food Codex and WHO limits for human consumption in the edible parts of fish samples and posed a risk for human health, Rajeshkumar and Munuswamy (2011) reported that Zn in the muscle tissue of milk fish from less polluted and polluted sites of Kaattuppalli Island, India were found in the range of 0.233 and 0.324 μg g−1 respectively, Sobhanardakani et al. (2011b) reported that Zn in the muscle tissue of five fish species (Otolithes ruber, Pampus argenteus, Parastromateus niger, Scomberomorus commerson, Onchorynchus mykiss) ranged from 0.005-0.04 μg g−1. Dural et al. (2007) analyzed Zn concentration in the muscle tissue of three fish species (Dicentrarchus labrax, Sparus aurata and Mugil cephalus) from the Tuzla lagoon, Turkey and reported that Zn contents ranged from 8.27±41.50, 8.82±99.8 and 12.2±76.98 μg g−1 in autumn, winter and spring 2001 respectively. Agusa et al. (2005) reported that Zn in the muscle of 12 species of marine fish collected from coastal areas in Malaysia ranged from 16.4-1730.0 μg g−1. Cohen et al. (2001) reported that Zn in the muscle of six fish species caught from Mugu Lagoon, Malibu Lagoon and Ballona Wetlands in shoutern California (F. parvipinnis, A. affinis, G. mirabilis, L. armatus, M. galloprovincialis and T. californianus) ranged from 12.0-650.0 μg g−1. Mormede and Davies (2001) reported that Zn in the muscle tissue of monkfish (Lophius piscatorius), black scabbard (Aphanopus carbo), blue ling (Molva dyp terygia), blue whiting (Micromesistius poutassou) and hake (Merluccius merluccius) were obtained from the continental slope of Rockall Trough, west of Scotland ranged from 0.37-3.90 μg g−1.
Conclusion
The results from this study suggested that significant differences existed in the metal concentrations across three different fish species. Also, analytical data obtained from this study shows that the metal concentrations for the fishes were generally within the FAO/WHO, U.S. FDA and U.S. EPA recommended limits for fish (table 1). There is therefore there is no serious health risk associated with the consumption of the three studied metals in the fishes analyzed. Both low-risk groups (adolescents and adults) and high-risk groups (pregnant mothers and children) must, based on the results obtained, reduce their consumption of fish. Therefore more research and assessments of seafood quality is needed in many countries to provide more data and help safeguard the health of humans.
Conflict of Interests
Authors have no conflict of interests.
References
- Agusa T., Kunito T., Yasunaga G., Iwata H., Subramanian A., Ismail A., Tanabe Sh. Concentrations of trace elements in marine fish and its risk assessment in Malaysia. Marine Pollution Bulletin, 51: 896-911 (2005).
- Ashraf W., Seddigi A., Abulkibash A., Khalid M. Levels of selected metals in canned fish consumed in Kingdom of Saudi Arabia. Environmental Monitoring and Assessment, 117: 271-79 (2006).
- Aucoin J., Blanchard R., Billiot C. Trace metals in fish and sediments from Lake Boeuf, South Eastern Louisiana. Microchemical Journal, 62: 299-307 (1999).
- Barandier C., Tanguy S., Pucheu S., Boucher F., De-Leiris J. Effect of antioxidant trace elements on the response of cardiac tissue to oxidative stress. Annual New York Academy of Science, 874: 138-55 (1999).
- Bilandzˇic´ N., Dokic´ M., Sedak M. Metal content determination in four fish species from the Adriatic Sea. Food Chemistry, 124: 1005-10 (2011).
- Boadi N.O., Twumasi S.K., Badu M., Osei I. Heavy metal contamination in canned fish marketed in Ghana. American Journal of Scientific and Industrial Research, 2(6): 877-82 (2011).
- Canbek M., Temir A.D, Mustafa U., Gokhan B., Ozgur E., Naime A. Preliminary assessment of heavy metals in water and some cyprinidae species from the Porsuk River, Turkey. Journal of Applied Biological Sciences, 1(3): 91–95 (2007).
- Canli M. and Atli G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environmental Pollution, 121: 129-36 (2005).
- Cohen T., Que Hee Sh.S, Ambrose R.F. Trace metals in fish and invertebrates of three California Coastal Wetlands. Marine Pollution Bulletin, 42(3): 224-32 (2001).
- De-Lorgeril M., Salen P., Accominotti M., Cadau M., Steghens J.P., Boucher F. Dietary and blood antioxidants in patients with chronic heart failure. Insights into the potential importance of selenium in heart failure. European Journal of Heart Failure, 3(6): 661-69 (2001).
- Dural M., Lugal Goksu M.Z., Akif Ozak A. Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chemistry, 102: 415-21 (2007).
- Eboh L., Mepba H.D., Ekpo M.B. Heavy metal contaminants and processing effects on the composition, storage stability and fatty acid profiles of five common commercially available fish species in Oron Local Government, Nigeria. Food Chemistry, 97(3): 490-97 (2006).
- Evans D.W., Dedoo D.K., Hanson P.J. Trace element concentrations in fish livers, Implications of variations with fish size in pollution monitoring. Marine Pollution Bulletin, 26(6): 329-34 (1993).
- Fernandes C., Fontainhas-Fernandes A., Monteiro S.M., Salgado M.A. Changes in plasma electrolytes and gill histopathology in wild Liza saliens from the Esmoriz-Paramos coastal lagoon. Bulletin of Environmental Contamination and Toxicology, 79: 301-05 (2007).
- Food and Agriculture. Compilation of Legal Limits for Hazardous Substances in Fish and Fishery Products. FAO Fishery Circular, Rome, 5-100 (1983).
- Fracacio R., Verani N.F., Espíndola E.L., Rocha O., Rigolin-Sa O., Andrade C.A. Alterations on growth and gill morphology of Danio rerio Pisces, Ciprinidae exposed to the toxic sediments. Brazilian Archives of Biology Technology, 46: 685-95 (2003).
- Garcia-Santos S., Fontainhas-Fernandes A., Wilson J.M. Cadmium tolerance in the Nile tilapia Oreochromis niloticus following acute exposure: assessment of some ionoregulatory parameters. Environmental Toxicology, 21(6): 33-46 (2006).
- Ghaderi N. and Ghafouri A.M. Comparative assessment of natural (forest and range) versus manmade (agriculture and urbane) environment in lake Zarivar. Iranian J Forest and Range Protection Research, 4: 19-27 (2006).
- Goyer R.A. Biology and nutrition of essential elements. In Risk Assessment of Essential Elements. ILSI Press, Washington, DC, 13–19 (1994).
- Kagi J.H. and Schaffer A. Biochemistry of metallothionein. Biochemistry, 27: 8509-15 (1998).
- Hellawell J.M. Biological Indicators of Freshwater Pollution and Environmental Management. Elsevier Publications, Amsterdam, 546-54 (1986).
- Ikem A. and Egeibor N.O. Assessment of trace elements in canned fishes (mackerel, tuna, salmon, sardines and herrings) marketed in Georgia and Alabama (United States of America). Journal of Food Composition Analysis, 18: 771-87 (2005).
- Kalay M. and Canil M. Elimination of essential (Cu, Zn) and non-essential (Cd, Pb) metals from tissue of a freshwater fish Tilapia zilli. Turkish Journal of Zoology, 24: 429-36 (2000).
- Keskin Y., Baskaya R., Ozyaral O., Yurdun T., Luleci N.E., Hayran O. Cadmium, lead, mercury and copper in fish from the Marmara Sea, Turkey. Bulletin of Environmental Contamination and Toxicology, 78: 258-61 (2007).
- MAFF. Monitoring and surveillance of non-radioactive contaminants in the aquatic environment and activities regulating the disposal of wastes at sea, 1993. Aquatic Environment Monitoring Report No. 44. Directorate of Fisheries Research, Lowestoft, (1995).
- Mallatt J. Fish gill structural changes induced by toxicants and other irritants: a statistical review. Canadian Journal of Fish Aquatic Sciences, 42: 630-48 (1985).
- Monteiro S.M., Mancera J.M., Fontaínhas S-Fernandes A., Sousa M. Copper induced alterations of biochemical parameters in the gill and plasma of Oreochromis niloticus. Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology, 141: 375-83 (2005).
- Mormede S. and Davies I.M. Heavy metal concentrations in commercial deep-sea fish from the Rockall Trough. Continental Shelf Research, 21: 899-916 (2001).
- Nair M., Jayalakshmy K.V., Balachandran K.K., Joseph T. Bioaccumulation of toxic metals by fish in a semi-enclosed tropical ecosystem. Environmental Forensics, 7: 197-206 (2006).
- Padmini E. and Usha Rani M. Impact of seasonal variation on HSP70 expression quantitated in stressed fish hepatocytes. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, B151: 278-85 (2008).
- Perry S.F. and Laurent P. Environmental effects on fish gill structure and function. In: Rankin J.C., Jenson F.B., Editors. Fish Ecophysiology. Chapman and Hall, London, 231-64 (1993).
- Rajeshkumar S. and Munuswamy N. Impact of metals on histopathology and expression of HSP 70 in different tissues of Milk fish (Chanos chanos) of Kaattuppalli Island, South East Coast, India. Chemosphere, 83: 415-21 (2011).
- Rashed M.N. Monitoring of environmental heavy metals in fish from Nasser Lake. Environmental International, 27: 27-33 (2001).
- Romao S., Donatti L., Freitas M.O., Teixeira J., Kusma J. Blood parameter analysis and morphological alterations as biomarkers on the health of Hoplias malabaricus and Geophagus brasiliensis. Brazilian Archives of Biology Technology, 49: 441-48 (2006).
- Salehifar E., Shokrzadeh M., Ghaemian A., Aliakbari S., Saeedi Saravi S.S. The study of Cu and Zn serum levels in idiopathic dilated cardiomyopathy (IDCMP) patients and its comparison with healthy volunteers. Biological Trace Element Research, 125(2): 97-108 (2008).
- Sankar T.V., Zynudheen A.A., Anandan R., Viswanathannair P.G. Distribution of organochlorine pesticides and heavy metal residues in fish and shellfish from Calicut region, Kerala, India. Chemosphere, 65: 583-90 (2006).
- Shokrzadeh M., Ghaemian A., Salehifar E., Aliakbari S., Saeedi Saravi S.S., Ebrahimi P. Serum zinc and copper levels in ischemic cardiomyopathy. Biological Trace Element Research, 127(2): 116-23 (2009).
- Sivaperumal P., Sankar T.V., Viswanathan Nair P.G. Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-a-vis international standards. Food Chemistry, 102: 612-20 (2007).
- Sobhanardakani S., Tayebi L., Farmany A., Cheraghi M. Analysis of trace elements (Cu, Cd and Zn) in muscle, gill and liver tissues of some fish species using anodic stripping voltammetry. Environmental Monitoring and Assessment, 184(11): 6607-11 (2011b).
- Sobhanardakani S., Tayebi L., Farmany A. Toxic metal (Pb, Hg and As) contamination of muscle, gill and liver tissues of Otolithes rubber, Pampus argenteus, Parastromateus niger, Scomberomorus commerson and Onchorynchus mykiss. World Applied Sciences Journal, 14(10): 1453-56 (2011a).
- Suppin D., Zahlbrucker R., Krapfenbauer -Cermak C.H., Hassan-Hauser C.H., Smulders F.J.M. Mercury, lead and cadmium content of fresh and canned fish collected from Austrian retail operations. Nutrition, 29: 456-60 (2005).
- Tabari S., Saravi S.S., Bandany G.A., Dehghan A., Shokrzadeh M. Heavy metals (Zn, Pb, Cd and Cr) in fish, water and sediments sampled form Southern Caspian Sea, Iran. Toxicology and Industrial Health, 26(10): 649-56 (2010).
- Tapia J., Duran E., Pena-Cortes F., Hauenstein E., Bertran C., Schlatter R. Micropogonias manni as a bioindicator for copper in Lake Budi (IX Region, Chile). Journal of the Chilean Chemical Society, 51: 901-04 (2006).
- Tepe Y., Turkmen M., Turkmen A. Assessment of heavy metals in two commercial fish species of four Turkish seas. Environmental Monitoring and Assessment, 146(1-3): 277-84 (2007).
- Topuzoglu G., Erbay A.R., Karul A.B., Yensel N. Concentrations of copper, zinc, and magnesium in sera from patients with idiopathic dilated cardiomyopathy. Biological Trace Element Research, 95(1): 7-11 (2003).
- Turkmen A., Turkmen M., Tepe Y., Mazlum Y., Oymael S. Heavy metal levels in Blue Crab (Callinectes sapidus) and Mullet (Mugil cephalus) in_Iskenderun Bay (North Eastern Mediterranean, Turkey). Bulletin of Environmental Contamination and Toxicology, 77: 186-93 (2006).
- Turkmen M., Turkmen A., Tepe Y., Ates A., Gokkus K. Determination of metal contaminations in sea foods from Marmara, Aegean and Mediterranean seas: Twelve fish species. Food Chemistry, 108: 794-800 (2008).
- Turkmen M., Turkmen A., Tepe Y., Tore Y., Ates A. Determination of metals in fish species from Aegean and Mediterranean Seas. Food Chemistry, 113: 233-37 (2009).
- Wood C.M. Branchial ion and acid-base transfer in freshwater teleost fish: environmental hyperoxia as a probe. Physiological Zoology, 64: 68-102 (1991).
- World Health Organization (WHO). Health criteria other supporting information, Guidelines for Drinking Water Quality. 2nd Edition. WHO, Geneva, 318-88 (1996).
- Yilmaz F., Ozdemir N., Demirak A., Tuna A.L. Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Food Chemistry, 100: 830-35 (2007).
- Yilmaz F. The comparison of heavy metal concentrations (Cd, Cu, Mn, Pb, and Zn) in tissues of three economically important fish (Anguilla anguilla, Mugil cephalus and Oreochromis niloticus) inhabiting Köycegiz Lake-Mugla (Turkey). Turkish Journal of Science & Technology, 4(1): 7-15 (2009).







